Highlights
-
•
We examined resting state connectivity in the DMN in cannabis users and controls.
-
•
Cannabis users displayed abnormal connectivity compared to healthy controls.
-
•
Abnormal connectivity in cannabis users related to poorer cognitive performance.
-
•
Abnormal brain communication may remain after ≥ 3 weeks of cannabis abstinence.
Keywords: Default mode network, Resting state functional connectivity, Cannabis, Adolescent, Young adult, Cognition
Abstract
Introduction
Cannabis is the most commonly used illicit substance in the United States, and nearly 1 in 4 young adults are current cannabis users. Chronic cannabis use is associated with changes in resting state functional connectivity (RSFC) in the default mode network (DMN) in adolescents and young adults; results are somewhat inconsistent across studies, potentially due to methodological differences. The aims of the present study were to examine potential differences in DMN RSFC between cannabis users and controls, and to examine, as an exploratory analysis, if gender moderated any findings. We further examined whether differences in RSFC related to differences in performance on selected neuropsychological measures.
Materials and methods
Seventy-seven 16–26-year-old participants underwent an MRI scan (including resting state scan), neuropsychological battery, toxicology screening, and drug use interview. Differences in DMN connectivity were examined between groups (cannabis vs. control) and with an exploratory group by gender interaction, using a left posterior cingulate cortex (PCC) seed-based analysis conducted in AFNI.
Results
Cannabis users demonstrated weaker connectivity than controls between the left PCC and various DMN nodes, and the right Rolandic operculum/Heschl’s gyrus. Cannabis users demonstrated stronger connectivity between the left PCC and the cerebellum and left supramarginal gyrus. The group by gender interaction was not significantly associated with connectivity differences. Stronger left PCC—cerebellum connectivity was associated with poorer performance on cognitive measures in cannabis users. In controls, intra-DMN connectivity was positively correlated with performance on a speeded selective/sustained attention measure.
Discussion
Consistent with our hypotheses and other studies, cannabis users demonstrated weaker connectivity between the left PCC and DMN nodes. Chronic THC exposure may alter GABA and glutamate concentrations, which may alter brain communication. Future studies should be conducted with a larger sample size and examine gender differences and the mechanism by which these differences may arise.
1. Introduction
Cannabis is the most commonly used illicit substance in the United States (Miech et al., 2020). In 2018, 24.1% of young adults 19–28 reported using cannabis within the past 30 days, while 8.0% reported using daily (Schulenberg et al., 2019). Given that the average age of initiation of cannabis use is in adolescence (X. Chen et al., 2017, Clark et al., 2013, Richmond-Rakerd et al., 2017), understanding the effects of cannabis on the developing brain is imperative.
The psychoactive component of cannabis is Δ9-tetrahyrdocannabinol (THC; Gaoni and Mechoulam, 1964, Gaoni and Mechoulam, 1971, Howlett et al., 2002), which is a partial agonist (Howlett et al., 2002) at cannabinoid receptors, type 1 (CB1Rs; Herkenham et al., 1990, Sim-Selley, 2003). CB1Rs are widely distributed in the cortex and subcortical structures (Glass et al., 1997, Herkenham et al., 1990, Mackie, 2005). As part of the retrograde-messenger endocannabinoid system, CB1Rs modulate release of many neurotransmitters, including glutamate and GABA (Pertwee, 2008, Wilson and Nicoll, 2002). Chronic THC exposure can lead to desensitization (Breivogel et al., 1999) and downregulation of CB1Rs (Breivogel et al., 1999, Oviedo et al., 1993, Rodriguez de Fonseca et al., 1994) and disruption of this normal modulatory activity, causing abnormal neurotransmitter levels (Howlett et al., 2002, Pertwee, 2008, Renard et al., 2018, Wilson and Nicoll, 2002), which may cause communication changes between brain regions and networks (Caballero & Tseng, 2012). However, downregulation of these receptors can reverse rapidly with abstinence from cannabis (D’Souza et al., 2016). Chronic activation of CB1Rs is thought to mediate the disruptive effects of cannabis use on cognition and brain communication; this appears to be especially pronounced when cannabis use occurs during adolescence (Caballero and Tseng, 2012, Mizrahi et al., 2017).
Given the neurodevelopment that is occurring during adolescence and young adulthood (Giedd et al., 1999, Giorgio et al., 2010, Lebel and Beaulieu, 2011), the young brain appears particularly vulnerable to the effects of chronic THC exposure (Adriani & Laviola, 2004). Exposure to cannabinoids during development is associated with gray matter (Filbey et al., 2014, Gilman et al., 2014), white matter (Filbey et al., 2014, Medina et al., 2007), and subcortical structural (Cousijn et al., 2012, Maple et al., 2019) abnormalities (Batalla et al., 2013, Lisdahl et al., 2018), including in areas rich in CB1Rs (Mackie, 2005). Further, regular cannabis use is related to lower IQ and deficits in processing speed, attention, executive functioning, and memory in this age group (Lisdahl et al., 2013, Lisdahl et al., 2018, Lisdahl et al., 2014). However, despite the continued maturation of functional brain networks across the lifespan (Betzel et al., 2014, Power et al., 2010), few studies have examined the impact of chronic cannabis exposure on brain connectivity in adolescents and young adults.
The default mode network (DMN) is a functional brain network that is active when the brain is at rest (Greicius et al., 2003) and is associated with stimulus-independent (i.e., “mind wandering,” Mason et al., 2007) and self-referential (Gusnard et al., 2001, Harrison et al., 2008) thought, as well as attentional control (Small et al., 2003). The DMN largely finishes it development by late adolescence (Bluhm et al., 2008), and is composed of the posterior cingulate cortex (PCC), hippocampal formation, lateral temporal cortex, medial and lateral parietal cortex, precuneus, and medial prefrontal cortex (mPFC; Buckner et al., 2008, Fox et al., 2005, Greicius et al., 2003, Raichle et al., 2001). Resting state functional connectivity (RSFC) analyses show that activation in the DMN is anticorrelated with activation in the task-positive network (Fox et al., 2005, Fransson, 2005). Stronger intra-DMN connectivity, as well as stronger anticorrelation between the DMN and executive control network, are associated with better working memory task performance (Sala-Llonch et al., 2012, Sambataro et al., 2010, Whitfield-Gabrieli et al., 2018).
Notably, DMN areas overlap with areas rich in CB1Rs (Buckner et al., 2008, Fox et al., 2005, Glass et al., 1997, Greicius et al., 2003, Mackie, 2005, Raichle et al., 2001), and acute THC administration has been shown to alter brain connectivity in DMN regions (Bossong et al., 2013, Whitfield-Gabrieli et al., 2018). Critically, the adolescent brain is particularly sensitive to substance use, including cannabis (Adriani & Laviola, 2004). Thus, it is important to examine how chronic cannabis use in this age group relates to RSFC in the DMN, and potential downstream effects of these relationships.
Several studies have examined relationships between chronic cannabis use and DMN RSFC in adolescents and young adults, with inconsistent findings. Pujol et al. (2014) reported that compared to male controls, male cannabis users showed higher RSFC between a PCC seed and the bilateral ventral PCC, and lower connectivity between the seed and the left and right dorsal PCC/precuneus. The latter was associated with poorer verbal recall in cannabis users. RSFC alterations persisted after 1 month of abstinence. Filbey et al. (2018) examined the effects of chronic cannabis use in young adults and adults on brain network connectivity. After 3 days of abstinence, the cannabis group demonstrated lower RSFC in the posterior cingulate gyrus compared to controls. Similarly, Wetherill et al. (2015) found lower RSFC in the DMN between the PCC and temporal cortex, mPFC, cerebellum, and parahippocampus, and higher RSFC between the PCC and right anterior insula, in non-abstinent cannabis users compared to controls. Lastly, Osuch et al. (2016) compared RSFC in the DMN between adolescent/young adult controls and presumably non-abstinent cannabis users; cannabis use was associated with lower RSFC in the right mPFC (BA6), and higher RSFC in the right BA30, compared to controls. Additionally, early-onset users demonstrated higher RSFC in parts of the DMN and lower total and verbal IQ (Osuch et al., 2016). In summary, results of studies examining RSFC in the DMN between cannabis users and controls to date are inconsistent. In studies with older samples and that excluded (Wetherill et al., 2015) or allowed very light (Filbey et al., 2018) nicotine use in their cannabis groups, RSFC in the DMN is generally lower in cannabis users compared to controls. Studies with younger samples find higher or lower connectivity depending on the DMN region.
Differing methodologies may help explain these inconsistent findings. Importantly, these studies differ in gender distribution, age, and inclusion of nicotine use and psychiatric disorders, which are all associated with differences in DMN connectivity (Bluhm et al., 2008, Broyd et al., 2009, Filbey et al., 2018, Hahn et al., 2007, Hjelmervik et al., 2014, Sambataro et al., 2010, Wetherill et al., 2015). As such, these factors should be considered when examining associations between cannabis use and RSFC in the DMN. In the current study, we attempt to consider these factors by increasing the number of female participants; focusing in on the specific late adolescence/young adult neurodevelopmental period; carefully measuring patterns of cannabis use, nicotine use, and use of other substances using a validated calendar-based method (Timeline Follow-Back; TLFB; Sobell et al., 1979); and excluding for comorbid independent psychiatric disorders.
Further, to our knowledge, no study to date has examined differences in RSFC between male and female cannabis users and controls in any network, including the DMN. This may be particularly important as females generally demonstrate stronger connectivity within DMN areas (Alarcon et al., 2018, Bluhm et al., 2008, Hjelmervik et al., 2014) and appear to be more susceptible to receptor-level adverse effects of chronic THC exposure (Burston et al., 2010, Farquhar et al., 2019, Silva et al., 2015), although whether cognition is differentially impacted is less clear (Pope et al., 1997, Solowij et al., 2011, Tait et al., 2011).
Thus, the primary aim of the present study was to examine potential differences in DMN RSFC using a left PCC seed between cannabis users and controls, and our secondary aim was to test whether gender moderated any findings. Given the relationship between chronic cannabis use and cognitive deficits in young adults (e.g., Lisdahl et al., 2014), we sought to describe relationships among connectivity between the left PCC and significant clusters and performance on select neuropsychological measures in order to further interpret brain-behavior relationships. Although there are heterogenous methodologies between existing studies of RSFC in the DMN in cannabis users and controls, studies with limited nicotine use in their samples generally find lower DMN RSFC in cannabis users (Filbey et al., 2018, Wetherill et al., 2015). Thus, given the relatively low nicotine use in our sample, we hypothesized that cannabis users would exhibit lower RSFC between the left PCC and other DMN nodes. We additionally hypothesized that RSFC would be related to cognition, with stronger connectivity between the left PCC and DMN nodes related to better performance on selected measures, or stronger connectivity between the left PCC and areas typically anti-correlated with the DMN related to poorer performance on these measures.
2. Materials and methods
2.1. Participants
Participants include 77 young adults (35 females, 42 males) from a larger neuroimaging study (PI: Lisdahl, R01DA030354). The Institutional Review Boards at the University of Wisconsin-Milwaukee and the Medical College of Wisconsin approved all protocols. Inclusion criteria included age 16–26, right-handedness, willingness to maintain abstinence from substances for the duration of the study; for the cannabis group: >40 past year cannabis uses, or significant (500 + uses) lifetime history of cannabis use with at least monthly current use; and for the control group: ≤ 20 lifetime uses of cannabis and ≤ 5 past year uses. Exclusion criteria included MRI contraindications, pregnancy, left-handedness, birth complications or premature birth (<33 weeks gestation), major medical or neurologic disorders, diabetes, hypertension, hyperlipidemia, hearing or vision impairment, learning or intellectual disability, head injury with loss of consciousness > 2 min, DSM-IV-TR Axis I disorders independent of substance use, current use of psychotropic medication, heavy other drug use (>25 lifetime uses of substances other than cannabis), use of > 10 cigarettes per day, failure to maintain abstinence at Session 4 or 5 (blood alcohol concentration of > 0.000, positive or increasing continuous sweat patch testing and/or urine toxicology), and the Wide Range Achievement Test-4th Edition (WRAT-4; Wilkinson, 2006) Reading t-score < 80. Eligible participants were divided into cannabis users (n = 37, 13 female) and controls (n = 40, 22 female).
2.2. Procedure
Individuals were recruited through flyers and advertisements posted in the community. After receiving verbal consent (or, if under 18, participant’s verbal assent and their parent’s verbal consent), interested potential participants were screened by phone for basic eligibility criteria. Those who remained eligible were mailed a written consent form (or an assent form if < 18, plus parent consent) prior to a detailed phone screen to assess comprehensive lifetime substance use (Customary Drinking and Drug Use Record, CDDR; Brown et al., 1998, STEWART and BROWN, 1995) and psychiatric history (Mini International Neuropsychiatric Interview, MINI; Sheehan et al., 1998, or MINI-Kid; Sheehan et al., 2010). For further detail, see Wallace et al., 2020a, Wallace et al., 2020b, Sullivan et al., 2020.
After obtaining informed consent, all participants completed 5 study sessions. Sessions 1–3 were each conducted one week apart; in each session, participants completed a brief neuropsychological battery (not used in the present study), mood battery (depression questionnaire at all sessions and state anxiety questionnaire at Session 1), and drug toxicology testing via a breath sample, urine screen, and continuous sweat patch testing (patch analyzed and changed weekly). At Session 4 (one week after Session 3), participants completed a longer neuropsychological battery, psychological questionnaires (including depression and state anxiety questionnaires), VO2 max treadmill testing, and drug testing (breath sample, urine toxicology, and collection of final sweat toxicology patch); the continuous sweat patch testing was concluded at this visit. At Session 5 (within 24–48 h of Session 4), participants completed a brain MRI scan and questionnaires, and provided breath and urine samples to verify abstinence from substance use.
2.3. Measures
At each study visit, participants provided a urine sample which was examined for adulterants (Specimen Validity Test; DrugTestStrips, Greenville, SC) and tested for cotinine (a nicotine metabolite; NicAlert strips, Nymox Pharmaceutical Corporation, Hasbrouck Heights, NJ), recent drug use (One Step Drug Screen Test Dip Card Panel; Innovacon, Inc., San Diego, CA), and (for females) pregnancy (HGC Pregnancy Test Card; DrugTestStrips, Greenville, SC). All participants completed a breath alcohol test (Alco-Sensor IV; Intoximeters Inc., St. Louis, MO). From Sessions 1–4, participants also wore a PharmCheck sweat patch that was changed at each visit, which monitored between-session substance use that may not be found in weekly urinalysis. See Sullivan et al. (2020) for further detail.
Past year substance use was measured with the Timeline Follow Back (TLFB; Sobell et al., 1979), a semi-structured measure in which participants are asked to recall their use of substances week-by-week over the past year using a calendar. Substances were measured using standard units (e.g., joints, and concentrates converted to joints [cannabis], standard drinks [alcohol], cigarettes). Lifetime substance use was measured with the CDDR (Brown et al., 1998, STEWART and BROWN, 1995).
2.4. Neuropsychological assessments
The following neuropsychological assessments were selected from the battery administered at Session 4 to be used in the present study. Estimated verbal intelligence and quality of education were assessed using the WRAT-4 (Wilkinson, 2006) Reading age-scaled score. Selective and sustained attention were measured with the Ruff 2 & 7 Total Speed and Total Accuracy raw scores (Ruff & Allen, 1996). Working memory and sustained attention were assessed with the Paced Auditory Serial Addition Test (PASAT; Gronwall, 1977) total correct score. The D-KEFS Color-Word Interference test Condition 3 (Inhibition) total completion time was used to assess inhibitory control (Delis et al., 2001). Verbal learning and memory were assessed with the California Verbal Learning Test, 2nd Edition (CVLT-II) Trial 1, Total Learning (Trials 1–5), and Long Delay Free Recall (LDFR) raw scores (Delis et al., 2000).
The Beck Depression Inventory-II (BDI-II; Beck, Steer, & Brown, 1996) was collected at Sessions 1–4, while the State-Trait Anxiety Inventory (state anxiety only; Spielberger, 1983) was collected at Sessions 1 and 4.
2.5. Neuroimaging
2.5.1. Acquisition parameters
Participants were scanned on a 3 T MR scanner (GE Healthcare, Waukesha, WI) at Medical College of Wisconsin. 3-dimensional, T-1 weighted anatomical images were obtained using a spoiled gradient-recalled at steady-state (SPGR) pulse sequence (TE = 3.4 s, TR = 8.2 s, TI = 450 ms, flip angle = 12 FOV = 240 mm, resolution = 256x256mm, slice thickness = 1 mm, 150 sagittal slices). An 8-minute resting state fMRI scan was conducted with the following parameters: TE = 25 ms, TR = 2 s, flip angle = 77 FOV = 240 mm, matrix = 64x64, slice thickness = 3.7 mm, 40 sagittal slices, 240 repetitions. During the resting state scan, participants were instructed to lie awake with their eyes closed.
2.5.2. Processing
Structural images underwent pre-processing using scripts from the 1000 Functional Connectomes Project (Fcon1000; Biswal et al., 2010), which call upon programs from, primarily, Analysis of Functional NeuroImages (AFNI; Cox, 1996, Cox, 2012), and FMRIB Software Library (FSL; Woolrich et al., 2009) software. Pre-processing steps included reorientation, skull stripping, segmentation into white and gray matter structures, registration to MNI space, and white matter segmentation. Raw functional images were pre-processed using Fcon1000 scripts (Biswal et al., 2010) including dropping the first 4 TRs, reorientation, motion correction to average of the time series, skull stripping, registration within each subject, registration to the anatomical image and to MNI space, spatial smoothing with a 6 mm FWHM Gaussian kernel, grand-mean scaling, band-pass filtering (high pass cutoff = 0.005 Hz, low pass cutoff = 0.1 Hz), linear and quadratic detrending, and regression of nuisance variables (including 6 motion parameters, global signal, white matter, and CSF).
2.6. Data analysis
Potential group differences on demographic and substance use variables between groups were examined using Chi-squares and ANOVAs with Tukey’s HSD post-hoc tests in SPSS v.25, after dividing participants into male cannabis users (n = 24), female cannabis users (n = 13), male controls (n = 18), and female controls (n = 22). Past year alcohol drinks and Session 5 cotinine were included as covariates in the general linear model (GLM) in AFNI as they differed significantly by group (see Table 1). A whole-brain correlation analysis was conducted with a 3 mm spherical seed in the left PCC at MNI (x,y,z) coordinates (−3, −50, 36; Ernst et al., 2019); this region was selected to be consistent with other studies of the DMN (Ernst et al., 2019, Fox et al., 2005). Using an Fcon1000 script (Biswal et al., 2010), the BOLD timeseries was extracted from the left PCC for each subject using 3dROIstats and then correlated with each voxel in the brain using 3dfim+; these correlations were transformed using Fischer’s Z-transform. The resultant seed-based connectivity maps for each subject were subsequently used in comparison of cannabis vs control groups using a GLM (Bijsterbosch et al., 2017). To correct for multiple comparisons, 10,000 Monte Carlo simulations were run within AFNI’s 3dClustSim. Using voxelwise (p < .001) and cluster-level (p < .05) thresholds, clusters with a minimum of 8 voxels were identified as significant.
Table 1.
Demographic and Drug Use Information PY = Past Year.
| M (SD) [Range] | Male Cannabis Users (n = 24) | Female Cannabis Users (n = 13) | Male Controls (n = 18) | Female Controls (n = 22) | p |
|---|---|---|---|---|---|
| Age | 21.71 (2.27) [17–26] | 21.62 (2.26) [19–25] | 20.89 (2.91) [16–25] | 21.09 (2.49) [16–25] | 0.69 |
| Ethnicity (% Caucasian) | 66.67% | 53.85% | 72.22% | 72.73% | 0.10 |
| Years of Education | 14.04 (1.71) [11–18] | 14.31 (1.49) [12–17] | 14.39 (2.77) [9–19] | 14.32 (1.91) [11–18] | 0.95 |
| WRAT-4 Reading Score | 109.25 (13.93) [80–133] | 100.77 (7.00) [93–120] | 107.89 (8.58) [92–126] | 104.64 (10.68) [87–133] | 0.12 |
| BDI-II Score | 5.04 (4.47) [0–19] | 6.38 (5.11) [1–18] | 3.39 (3.90) [0–10] | 2.23 (2.45) [0–8] | .02a |
| Session 1 STAI-State Score | 28.58 (5.75) [21–44] | 31.23 (7.14) [20–45] | 25.78 (6.45) [20–46] | 28.32 (7.38) [20–51] | 0.17 |
| Session 4 STAI-State Score | 26.13 (4.78) [20–36] | 26.69 (7.72) [20–43] | 26.72 (5.37) [20–39] | 26.41 (7.35) [20–42] | 0.99 |
| PY Cannabis Use (Joints + Conc) | 475.95 (511.07) [24–2306] | 260.60 (257.32) [13–879] | 0.82 (1.62) [0–5] | 0.05 (0.21) [0–1] | <.001b |
| Lifetime Cannabis Use (Joints) | 1433.50 (1581.92) [125–6000] | 837.23 (583.33) [101–2314] | 2.33 (4.97) [0–20] | 2.52 (5.11) [0–20] | <.001b |
| Length of Abstinence from Cannabis at MRI Scan | 37.00 (28.69) [18–151] | 29.54 (10.53) [20–58] | 151.20 (139.66) [32–332] | 260.00 (--) [260–260] (N = 1) | <0.001 |
| Age Cannabis Use Onset | 15.88 (2.15) [12–20] | 15.62 (2.22) [13–21] | 19.50 (1.76) [18–22] | 18.33 (2.12) [15–22] | <.001c |
| Age of Onset Regular Cannabis Use | 17.31 (1.90) [14–21] | 17.62 (1.56) [15–21] | ---- | ---- | 0.63 |
| PY Cigarettes | 253.81 (553.12) [0–1867] | 42.37 (68.35) [0–232] | 0.28 (0.46) [0–1] | 0.80 (2.64) [0–12] | .02d |
| Session 5 Cotinine Level | 2.17 (2.16) [0–6] | 1.23 (0.83) [0–3] | 1.00 (0.69) [0–3] | 1.18 (0.73) [0–3] | .03e |
| PY Alcohol Use (drinks) | 353.70 (304.29) [24–1120] | 221.58 (242.63) [37–883] | 158.08 (224.83) [0–698] | 68.43 (97.89) [0–450] | .001f |
aFemale Cannabis Users significantly higher than Female Controls. Male Cannabis Users marginally higher than Female Controls. bMale Cannabis Users significantly higher than Male and Female Controls. Female Cannabis Users marginally higher than Male and Female Controls. cMale and Female Cannabis Users significantly higher than Male and Female Controls. dMale Cannabis Users significantly higher than Female Controls, and marginally higher than Male Controls, p = .053. eMale Cannabis Users significantly higher than Male Controls, and marginally higher than Female Controls. fMale Cannabis Users significantly higher than Male and Female Controls.
AFNI’s 3dMVM (G. Chen et al., 2014) was used for the group analysis, identifying clusters significantly correlated with the left PCC seed by group and in a group*gender interaction. Data from significant clusters from either analysis were extracted using AFNI’s 3dROIstats and, using SPSS v.25, correlated with scores on selected neuropsychological measures in order to explore downstream cognitive effects of DMN connectivity differences.
3. Results
3.1. Demographic, mood, and drug use information
Demographic, mood, and drug use information is summarized in Table 1. Participants were divided into male cannabis users, male controls, female cannabis users, and female controls for the purposes of examination of group differences. Groups significantly differed in the Beck Depression Inventory-II (BDI-II) score (p = .02). Ethnicity was marginally different between groups (p = .10). Groups did not differ in age (p = .69), years of education (p = .95), WRAT-4 Reading score (p = .12), or state anxiety at Session 1 (p = .17) or Session 4 (p = .99).
Groups significantly differed in past year cannabis use (p < .001), lifetime cannabis use (p < .001), past year cigarettes (p = .02), past year alcohol use (p = .001), age of first cannabis use (p < .001), and cotinine level at Session 5 (p = .03). Post-hoc analysis found that, in summary, male cannabis users had higher past year and lifetime cannabis use than male and female controls; female cannabis users had marginally higher use than controls. Male and female cannabis users first initiated cannabis use at significantly younger ages than male and female controls (ps≤0.03). Male and female cannabis users did not differ in age of first (p = .99) or regular (p = .63) cannabis use. Male cannabis users had significantly higher cigarette consumption and Session 5 cotinine compared to male controls and marginally higher consumption and cotinine than female controls. Male, but not female, cannabis users consumed significantly more past year alcohol drinks compared to male (p = .04) and female (p < .001) controls. See Table 1 for further detail.
3.2. Primary findings
Participants were collapsed across gender to create cannabis and control groups for the main analysis. In both groups, the left PCC seed detected other core DMN nodes, including the PCC, precuneus, mPFC, lateral temporal cortex, parahippocampal gyrus, and parietal cortex/angular gyrus.
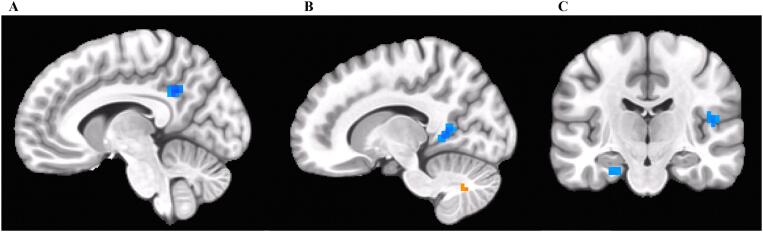
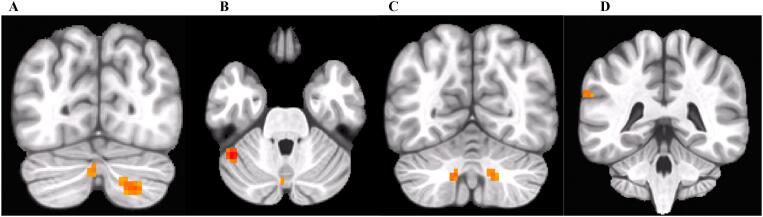
Controlling for recent cotinine and past year alcohol drinks, cannabis users displayed weaker connectivity compared to controls between the left PCC seed (at MNI (x,y,z) coordinates (−3, −50, 36)) and the right lingual gyrus/precuneus, left PCC/precuneus (at MNI (x,y,z) coordinates (−9, −51, 33)), right Rolandic operculum/Heschl’s gyrus, and left parahippocampal gyrus. Cannabis users displayed stronger connectivity compared to controls between the left PCC and the right cerebellum VII/Crus II, left cerebellum Crus I and Crus II, left cerebellum VIII, and left supramarginal gyrus. See Table 2 and Fig. 1, Fig. 2.
Table 2.
Significant Left PCC Connectivity Clusters.
| Location | # Voxels | MNI Coordinates(x,y,z) | Maximum t | |
|---|---|---|---|---|
| Main Effect of Group (CAN < CTL) | R Precuneus/R Lingual Gyrus | 33 | 12, −51, 6 | −4.08 |
| L PCC/L Precuneus | 21 | −9, −51, 33 | −4.30 | |
| R Rolandic Operculum/R Heschl’s Gyrus | 14 | 48, −18, 12 | −3.84 | |
| L Parahippocampal Gyrus | 12 | −21, −15, −24 | −3.72 | |
| Main Effect of Group (CAN > CTL) | R Cerebellum VII/Crus II | 51 | 24, −74, −45 | 4.17 |
| L Cerebellum Crus I | 27 | −45, −51, −30 | 4.84 | |
| L Cerebellum VIII | 13 | −12, −63, −39 | 4.09 | |
| L Supramarginal Gyrus | 11 | −66, −42, 30 | 3.92 | |
| L Cerebellum Crus II | 9 | −3, −75, –33 | 3.84 | |
| Main Effect of Gender (M > F) | R Temporal Pole | 10 | 54, 9, −18 | 3.73 |
| Session 5 Cotinine | R Cerebellum Crus I | 11 | 48, −72, −21 | −4.19 |
| Past Year Alcohol Drinks | R Precuneus | 9 | 21, −51, 24 | 4.03 |
Fig. 1.
Weaker connectivity (in blue) between the left PCC seed and A) left PCC/precuneus, B) right lingual gyrus/right precuneus (stronger connectivity is also seen between the left PCC and the right cerebellum VII/Crus II, pictured in orange; see Fig. 2), C) left parahippocampal gyrus (left) and right Rolandic operculum/Heschl’s gyrus (right) observed in cannabis users compared to controls. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 2.
Stronger connectivity (in orange) between the left PCC seed and A) the left cerebellum Crus II (left) and right cerebellum VII/Crus II (right), B) left cerebellum Crus I (left) and Crus II (right, see Fig. 2A), C) left cerebellum VIII (left) and right cerebellum VII/Crus II (right, see Fig. 2A), and D) left supramarginal gyrus observed in cannabis users compared to controls. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2.1. Exploratory gender analysis
The cannabis group*gender interaction was not significantly associated with any differences in connectivity between the left PCC and the rest of the brain. However, regardless of group status, male participants exhibited stronger left PCC—right temporal pole connectivity.
3.2.2. Covariate findings
Session 5 cotinine was negatively associated with left PCC—right cerebellum (Crus I) connectivity. Past year alcohol consumption was positively associated with stronger left PCC—right precuneus connectivity.
3.3. Brain-behavior relationships
Connectivity measurements from clusters that significantly differed by group were correlated with performance on selected neuropsychological measures. In cannabis users (Table 3), left PCC—left cerebellum Crus I connectivity was negatively correlated with PASAT total correct raw score (p = .04). Additionally, left PCC—left cerebellum VIII connectivity was negatively associated with the CVLT-II Total Learning (Trials 1–5) raw score (p = .04). In controls (Table 4), left PCC—left PCC/left precuneus connectivity was significantly positively correlated with performance on the Ruff 2 & 7 Total Speed raw score (p = .03).
Table 3.
Correlations Between Significant Clusters and Performance on Selected Neuropsychological Measures in Cannabis Users.
| R Crblm VII | R Lingual Gyr/R Precuneus | L Crblm (Crus I) | L PCC/L Precuneus | R RO/R Heschl’s Gyr | L Crblm VIII | L paraHC Gyr | L Supra-marginal Gyr | L Crblm (Crus II) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| PASAT Total Correct Raw Score | Pearson Correlation | −0.051 | 0.091 | -0.346* | 0.001 | −0.068 | 0.016 | −0.104 | −0.095 | 0.019 |
| Sig. (2-tailed) | 0.764 | 0.593 | 0.036 | 0.994 | 0.691 | 0.926 | 0.539 | 0.577 | 0.912 | |
| DKEFS Color-Word Interference Inhibition Condition Completion Time Raw Score | Pearson Correlation | 0.087 | −0.178 | −0.082 | 0.258 | −0.072 | 0.072 | 0.146 | 0.025 | 0.042 |
| Sig. (2-tailed) | 0.607 | 0.292 | 0.627 | 0.123 | 0.671 | 0.671 | 0.388 | 0.885 | 0.803 | |
| CVLT-II Trial 1 Raw Score | Pearson Correlation | −0.225 | 0.124 | −0.112 | 0.088 | 0.059 | −0.299 | −0.154 | −0.278 | −0.156 |
| Sig. (2-tailed) | 0.180 | 0.464 | 0.510 | 0.605 | 0.731 | 0.073 | 0.364 | 0.096 | 0.356 | |
| CVLT-II Total Correct (Trials 1–5) Raw Score | Pearson Correlation | −0.236 | 0.039 | −0.097 | 0.156 | 0.055 | -0.345* | −0.005 | −0.127 | −0.315 |
| Sig. (2-tailed) | 0.159 | 0.819 | 0.567 | 0.356 | 0.744 | 0.036 | 0.979 | 0.455 | 0.057 | |
| CVLT-II Long Delay Free Recall Raw Score | Pearson Correlation | −0.133 | −0.050 | 0.247 | 0.129 | −0.108 | −0.143 | −0.082 | −0.135 | −0.283 |
| Sig. (2-tailed) | 0.433 | 0.768 | 0.140 | 0.447 | 0.526 | 0.399 | 0.628 | 0.426 | 0.090 | |
| Ruff 2 & 7 Total Speed Raw Score | Pearson Correlation | −0.229 | −0.006 | −0.053 | 0.009 | −0.221 | −0.298 | 0.140 | −0.170 | −0.321 |
| Sig. (2-tailed) | 0.172 | 0.972 | 0.756 | 0.958 | 0.189 | 0.074 | 0.409 | 0.316 | 0.052 | |
| Ruff 2 & 7 Total Accuracy Raw Score | Pearson Correlation | 0.027 | 0.074 | 0.220 | 0.102 | 0.055 | 0.047 | −0.120 | −0.039 | 0.185 |
| Sig. (2-tailed) | 0.873 | 0.663 | 0.191 | 0.547 | 0.747 | 0.782 | 0.479 | 0.819 | 0.272 |
Table 4.
Correlations Between Significant Clusters and Performance on Selected Neuropsychological Measures in Controls.
| R Crblm VII | R Lingual Gyr/R Precuneus | L Crblm (Crus I) | L PCC/L Precuneus | R RO/R Heschl’s Gyr | L Crblm VIII | L paraHC Gyr | L Supra-marginal Gyr | L Crblm (Crus II) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| PASAT Total Correct Raw Score | Pearson Correlation | −0.119 | −0.101 | 0.013 | −0.003 | −0.152 | 0.133 | −0.176 | −0.026 | 0.027 |
| Sig. (2-tailed) | 0.466 | 0.536 | 0.938 | 0.984 | 0.351 | 0.415 | 0.277 | 0.872 | 0.868 | |
| DKEFS Color-Word Interference Inhibition Condition Completion Time Raw Score | Pearson Correlation | −0.033 | 0.028 | 0.052 | −0.009 | 0.283 | 0.020 | 0.117 | 0.102 | −0.217 |
| Sig. (2-tailed) | 0.842 | 0.862 | 0.748 | 0.956 | 0.077 | 0.901 | 0.472 | 0.533 | 0.179 | |
| CVLT-II Trial 1 Raw Score | Pearson Correlation | 0.147 | 0.047 | 0.116 | 0.109 | 0.101 | 0.304 | 0.215 | −0.054 | −0.106 |
| Sig. (2-tailed) | 0.365 | 0.772 | 0.477 | 0.504 | 0.536 | 0.056 | 0.183 | 0.738 | 0.514 | |
| CVLT-II Total Correct (Trials 1–5) Raw Score | Pearson Correlation | 0.041 | −0.006 | −0.166 | 0.005 | 0.097 | 0.066 | 0.033 | −0.094 | −0.039 |
| Sig. (2-tailed) | 0.803 | 0.969 | 0.305 | 0.974 | 0.553 | 0.684 | 0.842 | 0.564 | 0.812 | |
| CVLT-II Long Delay Free Recall Raw Score | Pearson Correlation | 0.065 | −0.071 | −0.157 | 0.081 | 0.034 | 0.046 | 0.190 | 0.002 | 0.051 |
| Sig. (2-tailed) | 0.689 | 0.663 | 0.334 | 0.618 | 0.833 | 0.776 | 0.241 | 0.988 | 0.754 | |
| Ruff 2 & 7 Total Speed Raw Score | Pearson Correlation | −0.140 | −0.137 | −0.184 | 0.342* | −0.086 | −0.087 | 0.000 | −0.100 | −0.089 |
| Sig. (2-tailed) | 0.389 | 0.401 | 0.255 | 0.031 | 0.596 | 0.595 | 0.999 | 0.541 | 0.585 | |
| Ruff 2 & 7 Total Accuracy Raw Score | Pearson Correlation | −0.089 | 0.033 | 0.042 | −0.243 | −0.238 | −0.039 | −0.242 | −0.276 | 0.046 |
| Sig. (2-tailed) | 0.584 | 0.841 | 0.797 | 0.130 | 0.138 | 0.813 | 0.132 | 0.085 | 0.780 | |
4. Discussion
The aims of the present study were to describe differences in RSFC in the DMN in adolescent and young adult cannabis users and controls and to explore if gender moderated any findings. Additionally, in a post-hoc exploratory analysis, we sought to examine brain-behavior relationships between DMN connectivity (in clusters that significantly differed by group) and performance on select neuropsychological measures in order to further interpret these findings. As stated above, typically, the DMN is activated at rest, and this is anti-correlated with activation in areas involved in task engagement (i.e., the task-positive network; Fox et al., 2005, Fransson, 2005). We found that adolescent/young adult cannabis users demonstrated weaker connectivity between the left posterior cingulate cortex (PCC) and the right lingual gyrus/precuneus, left PCC/precuneus, right Rolandic operculum/Heschl’s gyrus, and left parahippocampal gyrus, and stronger connectivity with the left supramarginal gyrus and portions of the cerebellum, compared to controls. In cannabis users, stronger connectivity between the left PCC and the cerebellum was correlated with poorer performance on sustained attention/working memory and verbal learning measures. In controls, stronger connectivity between the left PCC and the left PCC/precuneus was correlated with better speed on a selective and sustained attention measure. There were no significant interactions between group and gender in predicting left PCC connectivity.
Cannabis users exhibited weaker connectivity between the left PCC and other DMN nodes, such as the left PCC/left precuneus, left parahippocampal gyrus, right lingual gyrus/precuneus, and right Rolandic operculum/Heschl’s gyrus, compared to controls. Lesser intra-network connectivity (i.e., PCC, precuneus, parahippocampal gyrus) is consistent with our hypothesis, given the heavy CB1R presence in DMN regions and the low nicotine use in our sample, and with previous findings demonstrating lower connectivity in the PCC (Filbey et al., 2018), dorsal PCC/precuneus (Pujol et al., 2014), and parahippocampal gyrus (Wetherill et al., 2015) in cannabis users. In controls, connectivity within the DMN (left PCC—left PCC/precuneus) was significantly positively correlated with speed on a selective and sustained attention measure. This is broadly consistent with other work demonstrating that stronger intra-DMN connectivity is associated with better working memory task performance (Sala-Llonch et al., 2012, Sambataro et al., 2010).
Weaker connectivity was also seen between the left PCC and areas outside of the DMN, such as the right lingual gyrus and right Rolandic operculum/Heschl’s gyrus, in cannabis users compared to controls. Thus, the present findings suggest that cannabis users demonstrate abnormal connectivity between the PCC and sensory/perceptual associative areas (Blefari et al., 2017, Krumbholz et al., 2003, Mechelli et al., 2000). Acute THC administration induces altered perception (D'Souza et al., 2004), and alters activation in visual- and auditory-processing regions (including the lingual gyrus), which is associated with acute induction of psychotic symptoms (Winton-Brown et al., 2011). Chronic use is associated with abnormalities in these areas (Broyd et al., 2013, Hill et al., 2016) and in sensory gating in regular cannabis users. Similar disturbances are observed in schizophrenia (Edwards et al., 2009). Thus, it is possible that aberrant connectivity between the DMN and sensory/perceptual associative areas may be related to the unusual sensory experiences seen with cannabis use.
Contrary to other studies which found weaker connectivity between DMN and cerebellar areas in cannabis users in slightly older samples (Sweigert et al., 2020, Wetherill et al., 2015), we found that cannabis users demonstrated stronger connectivity between the left PCC and the cerebellum, specifically in the right cerebellum VII/Crus II, left cerebellum Crus I and II, and left cerebellum VIII in our adolescent/young adult sample. Studies have shown intrinsic connectivity between the DMN and Crus I, Crus II, and Lobule IX in healthy controls (Bernard et al. 2012, Krienen and Buckner, 2009, Wang et al., 2014). However, Crus I and II have also demonstrated functional connectivity with areas that are traditionally not considered part of the DMN, such as the dorsolateral prefrontal cortex (Fox et al., 2005, Krienen and Buckner, 2009) and executive control network (Habas et al., 2009). These areas are generally anticorrelated with the DMN (Di and Biswal, 2014, Fox et al., 2005, Greicius et al., 2003, Sridharan et al., 2008, Whitfield-Gabrieli et al., 2018, Whitfield-Gabrieli and Ford, 2012), so the current findings may relate to reduced anticorrelation between these networks. It is also possible that this stronger connectivity is a compensatory mechanism (Wall et al., 2019) due to downregulation of the CB1Rs (Breivogel et al., 1999, Sim-Selley, 2003, Sim-Selley and Martin, 2002) expressed in the cerebellum (Glass et al., 1997, Herkenham et al., 1990, Mackie, 2005, Nogueron et al., 2001). However, this is less likely given that recovery of CB1 receptors occurs rapidly after cessation of cannabis use (D’Souza et al., 2016), and participants in our sample had abstained from use for at least 3 weeks. In any case, chronic cannabis use appears to be associated with atypical DMN-cerebellar connectivity. Presently, stronger left PCC—cerebellum connectivity was negatively correlated with performance on measures of sustained attention/working memory and verbal learning in cannabis users, suggesting that stronger connectivity between these regions may have negative performance implications.
Cannabis users also displayed stronger connectivity between the left PCC and the left supramarginal gyrus. The supramarginal gyrus is negatively correlated with the PCC/precuneus in a small sample of healthy controls (Fransson, 2005), and may contribute modulatory activity between the DMN and the dorsal attention network (Di and Biswal, 2014). As part of the inferior parietal lobule, it may be considered a part of the task-positive network (Fox et al., 2005). Thus, it is possible that the higher left PCC—left supramarginal gyrus connectivity seen in cannabis users reflects lesser anticorrelation between the DMN and task-positive/attentional networks, which may relate to attentional lapses during task performance (Sonuga-Barke and Castellanos, 2007, Weissman et al., 2006).
As to possible mechanism underlying these findings, THC may disrupt the modulatory activity of the endocannabinoid system to alter PCC—DMN RSFC in chronic cannabis users. Chronic THC administration during adolescence may disrupt the optimal balance of excitatory (e.g., glutamate) and inhibitory (e.g., GABA) neurotransmitters (Renard et al., 2018), which may disrupt neural oscillations and affect neural communication, particularly in the gamma and beta bands (Edwards et al., 2009, Skosnik et al., 2012). (See Caballero and Tseng (2012) for review). CB1Rs are thought to facilitate oscillations in the gamma range (20–80 Hz; Wilson et al., 2001, Wilson and Nicoll, 2002) via GABAergic interneurons (Skosnik et al., 2016). Additionally, differences in GABA and glutamate concentrations relate to RSFC as examined using proton magnetic resonance spectroscopy. In healthy subjects, glutamate and GABA are positively and negatively (respectively) correlated with intrinsic functional connectivity in the DMN in healthy men (Kapogiannis et al., 2013). With chronic cannabis use, lower GABA and glutamate are seen in various brain regions of cannabis users relative to controls (Chang et al., 2006, Muetzel et al., 2013, Prescot et al., 2011, Prescot et al., 2013). Monthly cannabis use and dorsal ACC glutamate levels predict dorsal ACC--nucleus accumbens connectivity in young adults (Newman et al., 2020). To our knowledge, no study to date has directly examined the relationship between GABA concentrations and RSFC in cannabis users, nor has any study examined these relationships within the DMN, representing a future direction of interest. In summary, chronic cannabis use may disrupt CB1Rs’ modulatory activity of neurotransmitters such as GABA and glutamate (Howlett et al., 2002, Pertwee, 2008, Wilson and Nicoll, 2002), and this disruption may cause communication changes between brain regions and networks (Caballero & Tseng, 2012). However, this mechanism is solely hypothesis, and further study is needed to examine the relationships between chronic THC exposure, neurotransmitters, and RSFC in youth.
Given that, to our knowledge, no prior study has examined gender as a potential moderator of relationships between RSFC and cannabis use, we included a cannabis group by gender interaction as an exploratory analysis. In the present study, gender did not moderate RSFC between the left PCC and the rest of the brain. While it is certainly possible that our participants simply do not display differences in RSFC from the left PCC, it is worth noting that our study was underpowered to detect differences that only had a small effect size. Future studies should examine gender as a moderator using a larger sample.
While some recovery of cognitive function is seen with abstinence from cannabis in this age group (Lisdahl et al., 2013, Wallace et al., 2020a), it appears that subtle differences in communication between brain regions may persist in cannabis users even with 3 weeks of abstinence from cannabis (Sneider et al., 2008, Tapert et al., 2007, Wallace et al., 2020b). In cannabis users, these subtle communication differences may (present results; Wade et al., 2019) or may not (Sneider et al., 2008, Tapert et al., 2007, Wallace et al., 2020b) be associated with downstream behavioral differences. The adolescent brain is particularly sensitive to the effects of THC (Adriani & Laviola, 2004), and earlier exposure to substances is associated with poorer outcomes in cognition than typically seen in later-onset cannabis users. Thus, encouraging youth to minimize, eliminate, or delay their substance use onset until after age 18—via personalized feedback, psychoeducation, and exercise—may reduce some of the difficulties seen in early-onset users (Lisdahl et al., 2013).
The present study includes several limitations. The cross-sectional design precludes discussion of causality. Additionally, the sample size is relatively small, particularly of female cannabis users. There was a trend-level difference (p = .10) in ethnicity distribution between male and female cannabis users and controls. Notably, development of brain structures and networks, including the DMN, can vary across cultures (Dong et al., 2020). As such, cross-cultural neuropsychology needs greater research emphasis (Rivera Mindt et al., 2010), and results of the present study should be confirmed across socio-cultural groups that significantly differ from the current sample. Due to ongoing neurodevelopment, results may not generalize to other age groups (such as tween, early adolescent, middle-aged or older adults). The cannabis users present in this sample, on average, used cannabis a few times weekly to roughly daily, and the effects seen here may not generalize to lighter or heavier users. These data were collected prior to recent trends of vaping cannabis. Inhalation of cannabis vapor may be safer than inhalation of cannabis smoke from combustion (Giroud et al., 2015, Loflin and Earleywine, 2015), and as vaping of cannabis is increasing in youth (National Institute on Drug Abuse, 2019), future studies should examine effects of smoked and vaped cannabis on RSFC in the DMN. The resting state scan length was 8 min; reliability of data would likely improve with greater scan time (Anderson et al., 2011, Birn et al., 2013). Lastly, some of the differences in connectivity seen in the present study may be due to averaging error from connectivity differences in closely related anatomical areas (e.g., the right precuneus for the Heschl’s gyrus cluster; Bijsterbosch et al., 2017); larger samples may help increase reliability of findings. Prospective large-scale longitudinal studies such as the Adolescent Brain and Cognitive Development (ABCD) Study™ can address these concerns, as it includes a larger, more diverse sample in a prospective longitudinal design.
In summary, cannabis users demonstrated weaker RSFC between the left PCC and various DMN nodes, and stronger connectivity between the left PCC and the supramarginal gyrus and cerebellum. Stronger left PCC—left cerebellum connectivity was associated with poorer attention and working memory in cannabis users. These findings suggest that even after 3 weeks of monitored abstinence, brain communication remains abnormal in chronic cannabis users. Future studies should include a large sample and prospective longitudinal design to determine causality, and preclinical studies are needed to determine the underlying mechanisms.
CRediT authorship contribution statement
Megan M. Ritchay: Conceptualization, Methodology, Formal analysis, Writing - original draft, Writing - review & editing, Visualization, Investigation. Ashley A. Huggins: Formal analysis, Writing - review & editing. Alexander L. Wallace: Formal analysis, Writing - review & editing, Investigation. Christine L. Larson: Writing - review & editing, Supervision. Krista M. Lisdahl: Funding acquisition, Conceptualization, Methodology, Supervision, Project administration, Writing - review & editing.
Acknowledgements
We would like to thank Kristen Leer for her assistance with literature searching and proofreading.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2021.102664.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Adriani W., Laviola G. Windows of vulnerability to psychopathology and therapeutic strategy in the adolescent rodent model: Behav. Pharmacol. 2004;15(5):341–352. doi: 10.1097/00008877-200409000-00005. [DOI] [PubMed] [Google Scholar]
- Alarcon G., Pfeifer J.H., Fair D.A., Nagel B.J. Adolescent gender differences in cognitive control performance and functional connectivity between default mode and fronto-parietal networks within a self-referential context. Front. Behav. Neurosci. 2018;12:73. doi: 10.3389/fnbeh.2018.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J.S., Ferguson M.A., Lopez-Larson M., Yurgelun-Todd D. Reproducibility of single-subject functional connectivity measurements. AJNR Am. J. Neuroradiol. 2011;32(3):548–555. doi: 10.3174/ajnr.A2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batalla, A., Bhattacharyya, S., Yucel, M., Fusar-Poli, P., Crippa, J. A., Nogue, S., . . . Martin-Santos, R. (2013). Structural and functional imaging studies in chronic cannabis users: a systematic review of adolescent and adult findings. PLoS One, 8(2), e55821. doi:10.1371/journal.pone.0055821. [DOI] [PMC free article] [PubMed]
- Beck A.T., Steer R.A., Brown G.K. Psychological Corporation; San Antonio, TX: 1996. Manual for the Beck Depression Inventory-II. [Google Scholar]
- Bernard, J. A., Seidler, R. D., Hassevoort, K. M., Benson, B. L., Welsh, R. C., Wiggins, J. L., . . . Peltier, S. J. (2012). Resting state cortico-cerebellar functional connectivity networks: a comparison of anatomical and self-organizing map approaches. Front Neuroanat, 6, 31. doi:10.3389/fnana.2012.00031. [DOI] [PMC free article] [PubMed]
- Betzel R.F., Byrge L., He Y., Goni J., Zuo X.N., Sporns O. Changes in structural and functional connectivity among resting-state networks across the human lifespan. Neuroimage. 2014;102(Pt 2):345–357. doi: 10.1016/j.neuroimage.2014.07.067. [DOI] [PubMed] [Google Scholar]
- Bijsterbosch J., Smith S., Beckmann C. Oxford University Press; New York: 2017. Introduction to Resting State fMRI Functional Connectivity. [Google Scholar]
- Birn R.M., Molloy E.K., Patriat R., Parker T., Meier T.B., Kirk G.R., Nair V.A., Meyerand M.E., Prabhakaran V. The effect of scan length on the reliability of resting-state fMRI connectivity estimates. NeuroImage. 2013;83:550–558. doi: 10.1016/j.neuroimage.2013.05.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B.B., Mennes M., Zuo X.-N., Gohel S., Kelly C., Smith S.M., Beckmann C.F., Adelstein J.S., Buckner R.L., Colcombe S., Dogonowski A.-M., Ernst M., Fair D., Hampson M., Hoptman M.J., Hyde J.S., Kiviniemi V.J., Kotter R., Li S.-J., Lin C.-P., Lowe M.J., Mackay C., Madden D.J., Madsen K.H., Margulies D.S., Mayberg H.S., McMahon K., Monk C.S., Mostofsky S.H., Nagel B.J., Pekar J.J., Peltier S.J., Petersen S.E., Riedl V., Rombouts S.A.R.B., Rypma B., Schlaggar B.L., Schmidt S., Seidler R.D., Siegle G.J., Sorg C., Teng G.-J., Veijola J., Villringer A., Walter M., Wang L., Weng X.-C., Whitfield-Gabrieli S., Williamson P., Windischberger C., Zang Y.-F., Zhang H.-Y., Castellanos F.X., Milham M.P. Toward discovery science of human brain function. Proc. Natl. Acad. Sci. 2010;107(10):4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blefari M.L., Martuzzi R., Salomon R., Bello-Ruiz J., Herbelin B., Serino A., Blanke O., Foxe J. Bilateral Rolandic operculum processing underlying heartbeat awareness reflects changes in bodily self-consciousness. Eur. J. Neurosci. 2017;45(10):1300–1312. doi: 10.1111/ejn.13567. [DOI] [PubMed] [Google Scholar]
- Bluhm R.L., Osuch E.A., Lanius R.A., Boksman K., Neufeld R.W., Theberge J., Williamson P. Default mode network connectivity: effects of age, sex, and analytic approach. NeuroReport. 2008;19(8):887–891. doi: 10.1097/WNR.0b013e328300ebbf. [DOI] [PubMed] [Google Scholar]
- Bossong M.G., Jansma J.M., van Hell H.H., Jager G., Kahn R.S., Ramsey N.F. Default mode network effects of 9-tetrahydrocannabinol (THC) on human executive function. PLoS ONE. 2013;8(7) doi: 10.1371/journal.pone.0070074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breivogel C.S., Childers S.R., Deadwyler S.A., Hampson R.E., Vogt L.J., Sim-Selley L.J. Chronic delta9-tetrahydrocannabinol treatment produces a time-dependent loss of cannabinoid receptors and cannabinoid receptor-activated G proteins in rat brain. J. Neurochem. 1999;73(6):2447–2459. doi: 10.1046/j.1471-4159.1999.0732447.x. [DOI] [PubMed] [Google Scholar]
- Brown S.A., Myers M.G., Lippke L., Tapert S.F., Stewart D.G., Vik P.W. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): a measure of adolescent alcohol and drug involvement. J. Stud. Alcohol. 1998;59(4):427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Broyd S.J., Demanuele C., Debener S., Helps S.K., James C.J., Sonuga-Barke E.J.S. Default-mode brain dysfunction in mental disorders: A systematic review. Neurosci. Biobehav. Rev. 2009;33(3):279–296. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Broyd S.J., Greenwood L.-M., Croft R.J., Dalecki A., Todd J., Michie P.T., Johnstone S.J., Solowij N. Chronic effects of cannabis on sensory gating. Int. J. Psychophysiol. 2013;89(3):381–389. doi: 10.1016/j.ijpsycho.2013.04.015. [DOI] [PubMed] [Google Scholar]
- Buckner, R. L., Andrews-Hanna, J. R., & Schacter, D. L. (2008). The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci, 1124, 1-38. doi:10.1196/annals.1440.011. [DOI] [PubMed]
- Burston J.J., Wiley J.L., Craig A.A., Selley D.E., Sim-Selley L.J. Regional enhancement of cannabinoid CB1 receptor desensitization in female adolescent rats following repeated Delta-tetrahydrocannabinol exposure. Br. J. Pharmacol. 2010;161(1):103–112. doi: 10.1111/j.1476-5381.2010.00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero A., Tseng K.Y. Association of Cannabis Use during Adolescence, Prefrontal CB1 Receptor Signaling, and Schizophrenia. Front. Pharmacol. 2012;3:101. doi: 10.3389/fphar.2012.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L., Cloak C., Yakupov R., Ernst T. Combined and Independent Effects of Chronic Marijuana Use and HIV on Brain Metabolites. Jrnl NeuroImmune Pharm. 2006;1(1):65–76. doi: 10.1007/s11481-005-9005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Adleman N.E., Saad Z.S., Leibenluft E., Cox R.W. Applications of multivariate modeling to neuroimaging group analysis: a comprehensive alternative to univariate general linear model. NeuroImage. 2014;99:571–588. doi: 10.1016/j.neuroimage.2014.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Yu B., Lasopa S.O., Cottler L.B. Current patterns of marijuana use initiation by age among US adolescents and emerging adults: implications for intervention. Am. J. Drug Alcohol Abuse. 2017;43(3):261–270. doi: 10.3109/00952990.2016.1165239. [DOI] [PubMed] [Google Scholar]
- Clark T.T., Doyle O., Clincy A. Age of first cigarette, alcohol, and marijuana use among U.S. biracial/ethnic youth: a population-based study. Addict. Behav. 2013;38(9):2450–2454. doi: 10.1016/j.addbeh.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousijn J., Wiers R.W., Ridderinkhof K.R., van den Brink W., Veltman D.J., Goudriaan A.E. Grey matter alterations associated with cannabis use: results of a VBM study in heavy cannabis users and healthy controls. NeuroImage. 2012;59(4):3845–3851. doi: 10.1016/j.neuroimage.2011.09.046. [DOI] [PubMed] [Google Scholar]
- Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cox R.W. AFNI: What a long strange trip it's been. NeuroImage. 2012;62(2):743–747. doi: 10.1016/j.neuroimage.2011.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza D.C., Cortes-Briones J.A., Ranganathan M., Thurnauer H., Creatura G., Surti T., Planeta B., Neumeister A., Pittman B., Normandin M.D., Kapinos M., Ropchan J., Huang Y., Carson R.E., Skosnik P.D. Rapid changes in cannabinoid 1 receptor availability in cannabis-dependent male subjects after abstinence from cannabis. Biol. Psychiatry: Cognitive Neurosci. Neuroimaging. 2016;1(1):60–67. doi: 10.1016/j.bpsc.2015.09.008. [DOI] [PubMed] [Google Scholar]
- D'Souza D.C., Perry E., MacDougall L., Ammerman Y., Cooper T., Wu Y.-t., Braley G., Gueorguieva R., Krystal J.H. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacol. 2004;29(8):1558–1572. doi: 10.1038/sj.npp.1300496. [DOI] [PubMed] [Google Scholar]
- Delis D.C., Kaplan E., Kramer J.H. Psychological Corporation; San Antonio, TX: 2001. Delis-Kaplan Executive Functioning System Examiner's Manual. [Google Scholar]
- Delis D.C., Kramer J.H., Kaplan E., Ober B.A. The Psychological Corporation; San Antonio, TX: 2000. California Verbal Learning Test Second Edition Manual. [Google Scholar]
- Di, X., & Biswal, B. B. (2014). Modulatory interactions between the default mode network and task positive networks in resting-state. PeerJ, 2, e367. doi:10.7717/peerj.367. [DOI] [PMC free article] [PubMed]
- Dong H.-M., Castellanos F.X., Yang N., Zhang Z., Zhou Q., He Y.e., Zhang L., Xu T., Holmes A.J., Thomas Yeo B.T., Chen F., Wang B., Beckmann C., White T., Sporns O., Qiu J., Feng T., Chen A., Liu X., Chen X.u., Weng X., Milham M.P., Zuo X.-N. Charting brain growth in tandem with brain templates at school age. Science Bulletin. 2020;65(22):1924–1934. doi: 10.1016/j.scib.2020.07.027. [DOI] [PubMed] [Google Scholar]
- Edwards C.R., Skosnik P.D., Steinmetz A.B., O'Donnell B.F., Hetrick W.P. Sensory gating impairments in heavy cannabis users are associated with altered neural oscillations. Behav. Neurosci. 2009;123(4):894–904. doi: 10.1037/a0016328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M., Benson B., Artiges E., Gorka A.X., Lemaitre H., Lago T., Miranda R., Banaschewski T., Bokde A.L.W., Bromberg U., Brühl R., Büchel C., Cattrell A., Conrod P., Desrivières S., Fadai T., Flor H., Grigis A., Gallinat J., Garavan H., Gowland P., Grimmer Y., Heinz A., Kappel V., Nees F., Papadopoulos-Orfanos D., Penttilä J., Poustka L., Smolka M.N., Stringaris A., Struve M., van Noort B.M., Walter H., Whelan R., Schumann G., Grillon C., Martinot M.-L., Martinot J.-L. Pubertal maturation and sex effects on the default-mode network connectivity implicated in mood dysregulation. Transl. Psychiatry. 2019;9(1) doi: 10.1038/s41398-019-0433-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar C.E., Breivogel C.S., Gamage T.F., Gay E.A., Thomas B.F., Craft R.M., Wiley J.L. Sex, THC, and hormones: effects on density and sensitivity of CB1 cannabinoid receptors in rats. Drug Alcohol Depend. 2019;194:20–27. doi: 10.1016/j.drugalcdep.2018.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey F.M., Aslan S., Calhoun V.D., Spence J.S., Damaraju E., Caprihan A., Segall J. Long-term effects of marijuana use on the brain. PNAS. 2014;111(47):16913–16918. doi: 10.1073/pnas.1415297111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey F.M., Gohel S., Prashad S., Biswal B.B. Differential associations of combined vs. isolated cannabis and nicotine on brain resting state networks. Brain Struct. Funct. 2018;223(7):3317–3326. doi: 10.1007/s00429-018-1690-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., Raichle M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. U.S.A. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. Spontaneous low-frequency BOLD signal fluctuations: An fMRI investigation of the resting-state default mode of brain function hypothesis. Hum. Brain Mapp. 2005;26(1):15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaoni Y., Mechoulam R. Isolation, structure, and partial synthesis of an active constituent of Hashish. J. Am. Chem. Soc. 1964;86(8):1646–1647. [Google Scholar]
- Gaoni Y., Mechoulam R. Isolation and structure of delta-1-tetrahydrocannabinol and other neutral cannabinoids from hashish. J. Am. Chem. Soc. 1971;93(1):217–224. doi: 10.1021/ja00730a036. [DOI] [PubMed] [Google Scholar]
- Giedd J.N., Blumenthal J., Jeffries N.O., Castellanos F.X., Liu H., Zijdenbos A., Paus T., Evans A.C., Rapoport J.L. Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gilman J.M., Kuster J.K., Lee S., Lee M.J., Kim B.W., Makris N., van der Kouwe A., Blood A.J., Breiter H.C. Cannabis use is quantitatively associated with nucleus accumbens and amygdala abnormalities in young adult recreational users. J. Neurosci. 2014;34(16):5529–5538. doi: 10.1523/JNEUROSCI.4745-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio A., Watkins K.E., Chadwick M., James S., Winmill L., Douaud G., De Stefano N., Matthews P.M., Smith S.M., Johansen-Berg H., James A.C. Longitudinal changes in grey and white matter during adolescence. NeuroImage. 2010;49(1):94–103. doi: 10.1016/j.neuroimage.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Giroud C., de Cesare M., Berthet A., Varlet V., Concha-Lozano N., Favrat B. E-cigarettes: a review of new trends in cannabis use. Int. J. Environ. Res. Public Health. 2015;12(8):9988–10008. doi: 10.3390/ijerph120809988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass M., Faull R.L.M., Dragunow M. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience. 1997;77(2):299–318. doi: 10.1016/s0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- Greicius M.D., Krasnow B., Reiss A.L., Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. 2003;100(1):253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronwall D.M.A. Paced auditory serial-addition task: a measure of recovery from concussion. Percept. Mot. Skills. 1977;44(2):367–373. doi: 10.2466/pms.1977.44.2.367. [DOI] [PubMed] [Google Scholar]
- Gusnard D.A., Akbudak E., Shulman G.L., Raichle M.E. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc. Natl. Acad. Sci. 2001;98(7):4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas C., Kamdar N., Nguyen D., Prater K., Beckmann C.F., Menon V., Greicius M.D. Distinct cerebellar contributions to intrinsic connectivity networks. J. Neurosci. 2009;29(26):8586–8594. doi: 10.1523/JNEUROSCI.1868-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B., Ross T.J., Yang Y., Kim I., Huestis M.A., Stein E.A. Nicotine enhances visuospatial attention by deactivating areas of the resting brain default network. J. Neurosci. 2007;27(13):3477–3489. doi: 10.1523/JNEUROSCI.5129-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison B.J., Pujol J., Lopez-Sola M., Hernandez-Ribas R., Deus J., Ortiz H., Soriano-Mas C., Yucel M., Pantelis C., Cardoner N. Consistency and functional specialization in the default mode brain network. Proc. Natl. Acad. Sci. 2008;105(28):9781–9786. doi: 10.1073/pnas.0711791105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M., Lynn A.B., Little M.D., Johnson M.R., Melvin L.S., de Costa B.R., Rice K.C. Cannabinoid receptor localization in brain. Proc. Natl. Acad. Sci. 1990;87(5):1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill S.Y., Sharma V., Jones B.L. Lifetime use of cannabis from longitudinal assessments, cannabinoid receptor (CNR1) variation, and reduced volume of the right anterior cingulate. Psychiatry Research: Neuroimaging. 2016;255:24–34. doi: 10.1016/j.pscychresns.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelmervik, H., Hausmann, M., Osnes, B., Westerhausen, R., & Specht, K. (2014). Resting states are resting traits--an FMRI study of sex differences and menstrual cycle effects in resting state cognitive control networks. PLoS One, 9(7), e103492. doi:10.1371/journal.pone.0103492. [DOI] [PMC free article] [PubMed]
- Howlett A.C., Barth F., Bonner T.I., Cabral G., Casellas P., Devane W.A., Pertwee R.G. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 2002;54(2):161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- Kapogiannis D., Reiter D.A., Willette A.A., Mattson M.P. Posteromedial cortex glutamate and GABA predict intrinsic functional connectivity of the default mode network. NeuroImage. 2013;64:112–119. doi: 10.1016/j.neuroimage.2012.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krienen, F. M., & Buckner, R. L. (2009). Segregated fronto-cerebellar circuits revealed by intrinsic functional connectivity. Cereb Cortex, 19(10), 2485-2497. doi:10.1093/cercor/bhp135. [DOI] [PMC free article] [PubMed]
- Krumbholz K., Patterson R.D., Seither-Preisler A., Lammertmann C., Lutkenhoner B. Neuromagnetic evidence for a pitch processing center in Heschl's gyrus. Cereb. Cortex. 2003;13(7):765–772. doi: 10.1093/cercor/13.7.765. [DOI] [PubMed] [Google Scholar]
- Lebel C., Beaulieu C. Longitudinal development of human brain wiring continues from childhood into adulthood. J. Neurosci. 2011;31(30):10937–10947. doi: 10.1523/JNEUROSCI.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisdahl K.M., Gilbart E.R., Wright N.E., Shollenbarger S. Dare to delay? The impacts of adolescent alcohol and marijuana use onset on cognition, brain structure, and function. Front. Psychiatry. 2013;4:53. doi: 10.3389/fpsyt.2013.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisdahl K.M., Shollenbarger S., Sagar K.A., Gruber S.A. The Neurocognitive Impact of Alcohol and Marijuana Use on the Developing Adolescent and Young Adult Brain. In: Monti P.M., Colby S.M., Tevyaw T.O.L., editors. Brief Interventions for Adolescent Alcohol and Substance Abuse. The Guilford Press; New York: 2018. [Google Scholar]
- Lisdahl K.M., Wright N.E., Medina-Kirchner C., Maple K.E., Shollenbarger S. Considering cannabis: the effects of regular cannabis use on neurocognition in adolescents and young adults. Curr Addict Rep. 2014;1(2):144–156. doi: 10.1007/s40429-014-0019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loflin M., Earleywine M. No smoke, no fire: What the initial literature suggests regarding vapourized cannabis and respiratory risk. Can J Respir Ther. 2015;51(1):7–9. [PMC free article] [PubMed] [Google Scholar]
- Mackie K. Distribution of cannabinoid receptors in the central and peripheral nervous system. Handb Exp. 2005;Pharmacol(168):299–325. doi: 10.1007/3-540-26573-2_10. [DOI] [PubMed] [Google Scholar]
- Maple K.E., Thomas A.M., Kangiser M.M., Lisdahl K.M. Anterior cingulate volume reductions in abstinent adolescent and young adult cannabis users: association with affective processing deficits. Psychiatry Research: Neuroimaging. 2019;288:51–59. doi: 10.1016/j.pscychresns.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason M.F., Norton M.I., Van Horn J.D., Wegner D.M., Grafton S.T., Macrae C.N. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315(5810):393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechelli A., Humphreys G.W., Mayall K., Olson A., Price C.J. Differential effects of word length and visual contrast in the fusiform and lingual gyri during. Proc. R. Soc. Lond. B Biol. Sci. 2000;267(1455):1909–1913. doi: 10.1098/rspb.2000.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina K.L., Nagel B.J., Park A., McQueeny T., Tapert S.F. Depressive symptoms in adolescents: associations with white matter volume and marijuana use. J. Child Psychol. Psychiat. 2007;48(6):592–600. doi: 10.1111/j.1469-7610.2007.01728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miech R.A., Johnston L.D., O'Malley P.M., Bachman J.G., Schulenberg J.E., Patrick M.E. Volume I. Retrieved from; Secondary school students: 2020. (Monitoring the Future national survey results on drug use, 1975–2019:). http://monitoringthefuture.org/pubs.html#monographs. [Google Scholar]
- Mizrahi R., Watts J.J., Tseng K.Y. Mechanisms contributing to cognitive deficits in cannabis users. Neuropharmacology. 2017;124:84–88. doi: 10.1016/j.neuropharm.2017.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muetzel R.L., Marjańska M., Collins P.F., Becker M.P., Valabrègue R., Auerbach E.J., Lim K.O., Luciana M. In vivo 1H magnetic resonance spectroscopy in young-adult daily marijuana users. NeuroImage: Clinical. 2013;2:581–589. doi: 10.1016/j.nicl.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Drug Abuse. (2019, December 18, 2019). Vaping of marijuana on the rise among teens. Retrieved from https://www.drugabuse.gov/news-events/news-releases/2019/12/vaping-marijuana-rise-among-teens.
- Newman S.D., Cheng H.u., Kim D.-J., Schnakenberg-Martin A., Dydak U., Dharmadhikari S., Hetrick W., O’Donnell B. An investigation of the relationship between glutamate and resting state connectivity in chronic cannabis users. Brain Imaging Behav. 2020;14(5):2062–2071. doi: 10.1007/s11682-019-00165-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueron M.I., Porgilsson B., Schneider W.E., Stucky C.L., Hillard C.J. Cannabinoid receptor agonists inhibit depolarization-induced calcium influx in cerebellar granule neurons. J. Neurochem. 2001;79(2):371–381. doi: 10.1046/j.1471-4159.2001.00567.x. [DOI] [PubMed] [Google Scholar]
- Osuch E.A., Manning K., Hegele R.A., Théberge J., Neufeld R., Mitchell D., Williamson P., Gardner R.C. Depression, marijuana use and early-onset marijuana use conferred unique effects on neural connectivity and cognition. Acta Psychiatr. Scand. 2016;134(5):399–409. doi: 10.1111/acps.12629. [DOI] [PubMed] [Google Scholar]
- Oviedo A., Glowa J., Herkenham M. Chronic cannabinoid administration alters cannabinoid receptor binding in rat brain: a quantitative autoradiographic study. Brain Res. 1993;616(1-2):293–302. doi: 10.1016/0006-8993(93)90220-h. [DOI] [PubMed] [Google Scholar]
- Pertwee R.G. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br. J. Pharmacol. 2008;153(2):199–215. doi: 10.1038/sj.bjp.0707442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope H.G., Jr., Jacobs A., Mialet J.P., Yurgelun-Todd D., Gruber S. Evidence for a sex-specific residual effect of cannabis on visuospatial memory. Psychother. Psychosom. 1997;66(4):179–184. doi: 10.1159/000289132. [DOI] [PubMed] [Google Scholar]
- Power J.D., Fair D.A., Schlaggar B.L., Petersen S.E. The development of human functional brain networks. Neuron. 2010;67(5):735–748. doi: 10.1016/j.neuron.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescot A.P., Locatelli A.E., Renshaw P.F., Yurgelun-Todd D.A. Neurochemical alterations in adolescent chronic marijuana smokers: a proton MRS study. NeuroImage. 2011;57(1):69–75. doi: 10.1016/j.neuroimage.2011.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescot A.P., Renshaw P.F., Yurgelun-Todd D.A. gamma-Amino butyric acid and glutamate abnormalities in adolescent chronic marijuana smokers. Drug Alcohol Depend. 2013;129(3):232–239. doi: 10.1016/j.drugalcdep.2013.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol J., Blanco-Hinojo L., Batalla A., López-Solà M., Harrison B.J., Soriano-Mas C., Crippa J.A., Fagundo A.B., Deus J., de la Torre R., Nogué S., Farré M., Torrens M., Martín-Santos R. Functional connectivity alterations in brain networks relevant to self-awareness in chronic cannabis users. J. Psychiatr. Res. 2014;51:68–78. doi: 10.1016/j.jpsychires.2013.12.008. [DOI] [PubMed] [Google Scholar]
- Raichle M.E., MacLeod A.M., Snyder A.Z., Powers W.J., Gusnard D.A., Shulman G.L. A default mode of brain function. Proc. Natl. Acad. Sci. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard J., Rushlow W.J., Laviolette S.R. Effects of Adolescent THC Exposure on the Prefrontal GABAergic system: implications for schizophrenia-related psychopathology. Front. Psychiatry. 2018;9:281. doi: 10.3389/fpsyt.2018.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond-Rakerd L.S., Slutske W.S., Wood P.K. Age of initiation and substance use progression: a multivariate latent growth analysis. Psychol. Addict. Behav. 2017;31(6):664–675. doi: 10.1037/adb0000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera Mindt M., Byrd D., Saez P., Manly J. Increasing culturally competent neuropsychological services for ethnic minority populations: a call to action. Clin. Neuropsychologist. 2010;24(3):429–453. doi: 10.1080/13854040903058960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F., Gorriti M.A., Fernandez-Ruiz J.J., Palomo T., Ramos J.A. Downregulation of rat brain cannabinoid binding sites after chronic delta 9-tetrahydrocannabinol treatment. Pharmacol. Biochem. Behav. 1994;47(1):33–40. doi: 10.1016/0091-3057(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Ruff R.M., Allen C.C. Psychological Assessment Resources Inc; Odessa, FL: 1996. Ruff 2 & 7 Selective Attention Test. [Google Scholar]
- Sala-Llonch R., Peña-Gómez C., Arenaza-Urquijo E.M., Vidal-Piñeiro D., Bargalló N., Junqué C., Bartrés-Faz D. Brain connectivity during resting state and subsequent working memory task predicts behavioural performance. Cortex. 2012;48(9):1187–1196. doi: 10.1016/j.cortex.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Sambataro F., Murty V.P., Callicott J.H., Tan H.-Y., Das S., Weinberger D.R., Mattay V.S. Age-related alterations in default mode network: Impact on working memory performance. Neurobiol. Aging. 2010;31(5):839–852. doi: 10.1016/j.neurobiolaging.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulenberg, J. E., Johnston, L. D., O'Malley, P. M., Bachman, J. G., Miech, R. A., & Patrick, M. E. (2019). Monitoring the Future national survey results on drug use, 1975-2018: Volume II, College students and adults ages 19-60. Retrieved from http://monitoringthefuture.org/pubs.html#monographs.
- D.V. Sheehan Y. Lecrubier K.H. Sheehan P. Amorim J. Janavs E. Weiller G.C. Dunbar The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10 J Clin Psychiatry 59 Suppl 20 1998 22–33;quiz 34–57. [PubMed]
- Sheehan D.V., Sheehan K.H., Shytle R.D., Janavs J., Bannon Y., Rogers J.E., Milo K.M., Stock S.L., Wilkinson B. Reliability and Validity of the Mini International Neuropsychiatric Interview for Children and Adolescents (MINI-KID) J. Clin. Psychiatry. 2010;71(03):313–326. doi: 10.4088/JCP.09m05305whi. [DOI] [PubMed] [Google Scholar]
- Silva L., Harte-Hargrove L., Izenwasser S., Frank A., Wade D., Dow-Edwards D. Sex-specific alterations in hippocampal cannabinoid 1 receptor expression following adolescent delta-9-tetrahydrocannabinol treatment in the rat. Neurosci. Lett. 2015;602:89–94. doi: 10.1016/j.neulet.2015.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim-Selley L.J. Regulation of Cannabinoid CB1 Receptors in the Central Nervous System by Chronic Cannabinoids. Crit. Rev. Neurobiol. 2003;15(2):91–119. doi: 10.1615/critrevneurobiol.v15.i2.10. [DOI] [PubMed] [Google Scholar]
- Sim-Selley L.J., Martin B.R. Effect of chronic administration of R-(+)-[2,3-Dihydro-5-methyl-3-[(morpholinyl)methyl]pyrrolo[1,2,3-de]-1,4-benzoxaz inyl]-(1-naphthalenyl)methanone mesylate (WIN55,212–2) or delta(9)-tetrahydrocannabinol on cannabinoid receptor adaptation in mice. J. Pharmacol. Exp. Ther. 2002;303(1):36–44. doi: 10.1124/jpet.102.035618. [DOI] [PubMed] [Google Scholar]
- Skosnik P.D., Cortes-Briones J.A., Hajós M. It’s All in the Rhythm: The Role of Cannabinoids in Neural Oscillations and Psychosis. Biol. Psychiatry. 2016;79(7):568–577. doi: 10.1016/j.biopsych.2015.12.011. [DOI] [PubMed] [Google Scholar]
- Skosnik P.D., D'Souza D.C., Steinmetz A.B., Edwards C.R., Vollmer J.M., Hetrick W.P., O'Donnell B.F. The Effect of Chronic Cannabinoids on Broadband EEG Neural Oscillations in Humans. Neuropsychopharmacol. 2012;37(10):2184–2193. doi: 10.1038/npp.2012.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small D.M., Gitelman D.R., Gregory M.D., Nobre A.C., Parrish T.B., Mesulam M.-M. The posterior cingulate and medial prefrontal cortex mediate the anticipatory allocation of spatial attention. NeuroImage. 2003;18(3):633–641. doi: 10.1016/s1053-8119(02)00012-5. [DOI] [PubMed] [Google Scholar]
- Sneider J.T., Pope H.G., Jr., Silveri M.M., Simpson N.S., Gruber S.A., Yurgelun-Todd D.A. Differences in regional blood volume during a 28-day period of abstinence in chronic cannabis smokers. Eur. Neuropsychopharmacol. 2008;18(8):612–619. doi: 10.1016/j.euroneuro.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell L.C., Maisto S.A., Sobell M.B., Cooper A.M. Reliability of alcohol abusers' self-reports of drinking behavior. Behav. Res. Ther. 1979;17(2):157–160. doi: 10.1016/0005-7967(79)90025-1. [DOI] [PubMed] [Google Scholar]
- Solowij N., Jones K.A., Rozman M.E., Davis S.M., Ciarrochi J., Heaven P.C.L., Lubman D.I., Yücel M. Verbal learning and memory in adolescent cannabis users, alcohol users and non-users. Psychopharmacology. 2011;216(1):131–144. doi: 10.1007/s00213-011-2203-x. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke E.J.S., Castellanos F.X. Spontaneous attentional fluctuations in impaired states and pathological conditions: a neurobiological hypothesis. Neurosci. Biobehav. Rev. 2007;31(7):977–986. doi: 10.1016/j.neubiorev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Spielberger C.D. Mind Garden; Palo Alto, CA: 1983. Manual for the State-Trait Inventory STAI (Form Y) [Google Scholar]
- Sridharan D., Levitin D.J., Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc. Natl. Acad. Sci. 2008;105(34):12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEWART DAVID.G., BROWN SANDRA.A. Withdrawal and dependency symptoms among adolescent alcohol and drug abusers. Addiction. 1995;90(5):627–635. doi: 10.1046/j.1360-0443.1995.9056274.x. [DOI] [PubMed] [Google Scholar]
- Sullivan, R. M., Wallace, A. L., Wade, N. E., Swartz, A. M., & Lisdahl, K. M. (2020). Assessing the Role of Cannabis Use on Cortical Surface Structure in Adolescents and Young Adults: Exploring Gender and Aerobic Fitness as Potential Moderators. Brain Sci, 10(2). doi:10.3390/brainsci10020117. [DOI] [PMC free article] [PubMed]
- Sweigert J., Pagulayan K., Greco G., Blake M., Larimer M., Kleinhans N.M. A multimodal investigation of cerebellar integrity associated with high‐risk cannabis use. Addict. Biol. 2020;25(6) doi: 10.1111/adb.v25.610.1111/adb.12839. [DOI] [PubMed] [Google Scholar]
- Tait, R. J., Mackinnon, A., & Christensen, H. (2011). Cannabis use and cognitive function: 8-year trajectory in a young adult cohort. Addiction, 106(12), 2195-2203. doi:10.1111/j.1360-0443.2011.03574.x. [DOI] [PubMed]
- Tapert S.F., Schweinsburg A.D., Drummond S.P.A., Paulus M.P., Brown S.A., Yang T.T., Frank L.R. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology. 2007;194(2):173–183. doi: 10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade N.E., Wallace A.L., Swartz A.M., Lisdahl K.M. Aerobic fitness level moderates the association between cannabis use and executive functioning and psychomotor speed following abstinence in adolescents and young adults. J. Int. Neuropsychol. Soc. 2019;25(2):134–145. doi: 10.1017/S1355617718000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall M.B., Pope R., Freeman T.P., Kowalczyk O.S., Demetriou L., Mokrysz C., Hindocha C., Lawn W., Bloomfield M.AP., Freeman A.M., Feilding A., Nutt D., Curran H.V. Dissociable effects of cannabis with and without cannabidiol on the human brain’s resting-state functional connectivity. J. Psychopharmacol. 2019;33(7):822–830. doi: 10.1177/0269881119841568. [DOI] [PubMed] [Google Scholar]
- Wallace A.L., Maple K.E., Barr A.T., Lisdahl K.M. BOLD responses to inhibition in cannabis-using adolescents and emerging adults after 2 weeks of monitored cannabis abstinence. Psychopharmacology. 2020 doi: 10.1007/s00213-020-05608-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace A.L., Wade N.E., Lisdahl K.M. Impact of 2 Weeks of Monitored Abstinence on Cognition in Adolescent and Young Adult Cannabis Users. J Int Neuropsychol Soc. 2020;26(8):776–784. doi: 10.1017/S1355617720000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Zou F., Shao Y., Ye E., Jin X., Tan S., Hu D., Yang Z. Disruptive changes of cerebellar functional connectivity with the default mode network in schizophrenia. Schizophr. Res. 2014;160(1-3):67–72. doi: 10.1016/j.schres.2014.09.034. [DOI] [PubMed] [Google Scholar]
- Weissman D.H., Roberts K.C., Visscher K.M., Woldorff M.G. The neural bases of momentary lapses in attention. Nat. Neurosci. 2006;9(7):971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- Wetherill R.R., Fang Z., Jagannathan K., Childress A.R., Rao H., Franklin T.R. Cannabis, cigarettes, and their co-occurring use: Disentangling differences in default mode network functional connectivity. Drug Alcohol Depend. 2015;153:116–123. doi: 10.1016/j.drugalcdep.2015.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Fischer A.S., Henricks A.M., Khokhar J.Y., Roth R.M., Brunette M.F., Green A.I. Understanding marijuana's effects on functional connectivity of the default mode network in patients with schizophrenia and co-occurring cannabis use disorder: A pilot investigation. Schizophr. Res. 2018;194:70–77. doi: 10.1016/j.schres.2017.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Ford J.M. Default Mode Network Activity and Connectivity in Psychopathology. Annu. Rev. Clin. Psychol. 2012;8(1):49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- Wilkinson G. Wide Range Inc; Wilmington, DE: 2006. Wide Range Achievement Test, 4th Edition (WRAT-4) Manual. [Google Scholar]
- Wilson R.I., Kunos G., Nicoll R.A. Presynaptic Specificity of Endocannabinoid Signaling in the Hippocampus. Neuron. 2001;31(3):453–462. doi: 10.1016/s0896-6273(01)00372-5. [DOI] [PubMed] [Google Scholar]
- Wilson R.I., Nicoll R.A. Endocannabinoid signaling in the brain. Science. 2002;296(5568):678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- Winton-Brown T.T., Allen P., Bhattacharrya S., Borgwardt S.J., Fusar-Poli P., Crippa J.A., Seal M.L., Martin-Santos R., Ffytche D., Zuardi A.W., Atakan Z., McGuire P.K. Modulation of Auditory and Visual Processing by Delta-9-Tetrahydrocannabinol and Cannabidiol: an fMRI Study. Neuropsychopharmacol. 2011;36(7):1340–1348. doi: 10.1038/npp.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich M.W., Jbabdi S., Patenaude B., Chappell M., Makni S., Behrens T., Beckmann C., Jenkinson M., Smith S.M. Bayesian analysis of neuroimaging data in FSL. NeuroImage. 2009;45(1):S173–S186. doi: 10.1016/j.neuroimage.2008.10.055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.