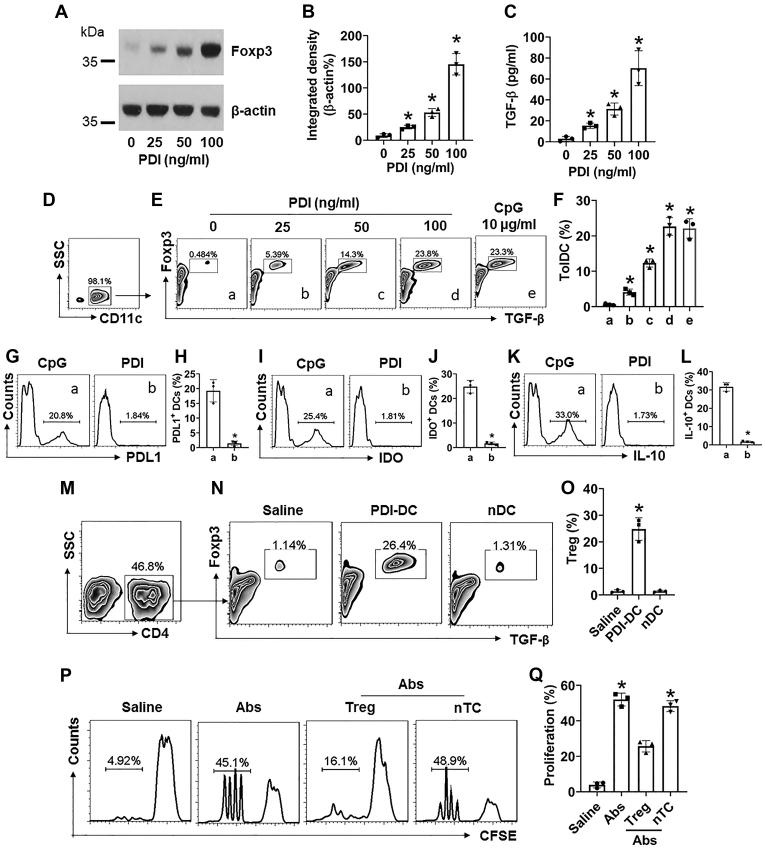
Figure 3.
PDI induces TolDCs. A–C, DEC205+ DCs were isolated from the human blood samples and cultured in the presence of PDI at gradient concentrations for 48 h. Immunoblots show Foxp3 protein in DCs (A); bars show integrated density of immunoblots of A (B); bars show TGF-β levels in DC culture supernatant (C). D, gated plots show the purity of DCs. E, gated plots show TolDC frequency. F, bars show summarized data of TolDC counts. G–L, DCs were cultured in the presence of CpG (10 μg/ml) or DPI (100 ng/ml) for 48 h. Gated histograms show the frequency of PDL1+ DCs (G), or IDO+ DCs (I), or IL-10+ DCs (K). Bars show summarized positive cell counts of PDL1 (H), or IDO (J), or IL-10 (L). M–O, DCs were primed by exposing to PDI (100 ng/ml) in the culture for 48 h and then cocultured with naïve CD4+ CD25¯ T cells (DC:T cell = 1:1) in the presence of IL-2 (20 ng/ml) for 72 h. nDC: Naïve DC (controls). M, CD4+ T cells were gated first. N, gated FCS plots show Foxp3+ TGF-β+ Treg frequency. O, bars show mean ± SEM of 3 experiments of panel N. P–Q, Tregs were prepared as described in panel M-O and cocultured with naïve CD4+ CD25¯ T cells (labeled with CFSE) with the conditions denoted above each subpanel of P. Gated histograms show CD4+ T cell proliferation (P). Q, bars show summarized CD4+ T cell proliferation. Ab: Anti-CD3/CD28 Ab (5 μg/ml of each Ab). nTC: Naïve CD4+ T cells (controls). The data of bars are presented as mean ± SEM. ∗, p < 0.01 (ANOVA with Dunnett’s test), compared with the dose 0 (B, C) or group a (F), or the saline group (O, Q). The data represent three independent experiments (each sample was analyzed in triplicate with average of the three readouts as one datum).