Fig. 2.
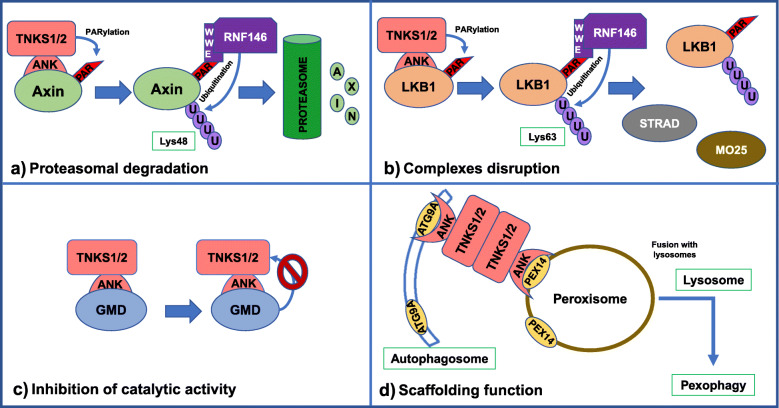
Biological responses triggered after the recognition of TNKS1/2 substrates. Tankyrases recognize their binding partners via the ANK domain. The most studied function of TNKS1/2 is the regulation of protein stability via proteasomal degradation. Tankyrases PARylate many substrates like Axin1/2, PTEN or 3BP2 and then, the E3 ubiquitin ligase RNF146 recognizes the PARylated proteins and adds a poly-ubiquitin chain linked by its Lys48 residues, which trigger their proteasomal degratation (a). More recently, LKB1 was shown to interact with TNKS1/2 and RNF146, but in this case, tankyrase is able to PARylate LKB1 and RNF146 adds a poly-ubiquitin chain linked by Lys63 in orde to disrupt the complex formed by LKB1, STRAD and MO25 (b). The interaction between tankyrase and the proteins GMD and Mcl-1 causes the inhibition of the catalytic activity of TNKS1/2 (c). Tankyrases also can work as scaffolding proteins via their ANK domain, promoting the interaction between different proteins like PEX14 and ATG9 (without PARylation) or MERIT40 (low levels of PAR) (d)