Abstract
Objectives
The aim of this study was to investigate the association between taste and smell losses and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, and to elucidate whether taste preference influences such taste loss.
Methods
A matched case–control study was conducted in 366 Thai participants, including 122 who were confirmed SARS-CoV-2-positive by RT-PCR (case group) and 244 who were SARS-CoV-2-negative (control group). Taste, smell, and appetite changes were assessed by self-reported visual analog scale. Preference for sweet, salty, umami, sour, bitter, and spicy were judged using the validated TASTE-26 questionnaire.
Results
Partial taste and smell losses were observed in both groups, while complete losses (ageusia and anosmia) were detected only in the case group. Moreover, only ageusia and anosmia were associated with SARS-CoV-2 positivity (P < 0.001, odds ratio of 14.5 and 27.5, respectively). Taste, smell, and appetite scores were more severely reduced in the case group (P < 0.0001). Multivariate analysis showed that anosmia and ageusia were the best predictors of SARS-CoV-2 positivity, followed by appetite loss and fever. Simultaneous losses of taste and smell but not taste preferences were associated with SARS-CoV-2 positivity (P < 0.01, odds ratio 2.28).
Conclusions
Complete, but not partial, losses of taste and smell were the best predictors of SARS-CoV-2 infection. During the current COVID-19 pandemic, healthy persons with sudden simultaneous complete loss of taste and smell should be screened for COVID-19.
Keywords: COVID-19, Taste, Smell, Appetite, Case–control study, Thai
Introduction
Coronavirus disease 2019 (COVID-19) is a pandemic respiratory infectious disease, with 110.7 million cases leading to over 2.4 million deaths reported globally on 23 February, 2021 (World Health Organization, 2020a). COVID-19 is caused by a novel pathogen, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (World Health Organization, 2020b). Early detection could help reduce the morbidity and mortality rates (Hashmi and Asif, 2020). Common symptoms of COVID-19 include a body temperature >37.5 °C and a dry cough in mild cases, fever, cough, abdominal discomfort, and abnormal blood biomarkers in moderate cases, and difficulty breathing and dyspnea in severe cases (Guan et al., 2020). Unfortunately, these symptoms are comparatively similar to those of other respiratory infectious diseases such as influenza (Hashmi and Asif, 2020). Therefore, there is a need to identify pathognomonic features of COVID-19.
Besides respiratory symptoms, alterations in gustatory and olfactory function are commonly found in COVID-19. A systemic review and meta-analysis reported that the overall prevalence of alterations in taste and smell senses among COVID-19 patients was 47% (Borsetto et al., 2020). Some patients have had reduced taste and smell senses (hypogeusia and hyposmia, respectively), while others have had complete losses of taste and smell (ageusia and anosmia) (Vaira et al., 2020). Interestingly, isolated sudden onset anosmia (ISOA) occurring separately from nasal obstruction or rhinitis has been variedly reported in 22–88% of SARS-CoV-2-infected individuals (Gane et al., 2020, Bagheri et al., 2020). The underlying mechanisms of the taste and smell losses in COVID-19 cases remain unclear. Existing evidence suggests that SARS-CoV-2 could bind to angiotensin converting enzyme 2 (ACE2), which is highly expressed in nasal mucosa (Sungnak et al., 2020). Since the anosmia in COVID-19 cases may occur without nasal obstruction, neurotoxicity of SARS-CoV-2 to olfactory and gustatory neurons has been proposed as a potential mechanism of taste and smell losses (Jafari et al., 2020).
Smell alteration can be caused by upper respiratory infections, allergic rhinitis, sinusitis, nasal polyps, head trauma, neurological disorders such as Parkinson’s disease, Alzheimer’s disease, psychiatric disorders such as major depression, medications, toxins, vitamin B12 deficiency, and autoimmune diseases such as systemic lupus erythematosus (Malaty and Malaty, 2020). Taste alteration can be caused by aging, zinc deficiency, viral infection, neurological diseases, radiation therapy, chronic alcohol consumption, smoking, nerve or brain trauma, and medications causing hyposalivation (Risso et al., 2020). Previous studies on taste and smell alterations in COVID-19 patients have mostly been of cross-sectional design. Thus, the observed association could be confounded by other factors. Currently, it is still unclear whether SARS-CoV-2 infection has a causal association with hypogeusia, ageusia, hyposmia, and anosmia (Tanasa et al., 2020).
People with a stronger preference for a certain taste often have a higher recognition threshold for that taste, i.e. a higher concentration of the taste substance is required to recognize the taste (Chamoun et al., 2019). A previous study in cancer patients receiving radiation therapy showed that patients with higher taste thresholds were more susceptible to taste loss than those with lower taste thresholds (Vittayakasemsont, 2016). Thus, it was speculated that people with a stronger taste preference may be more sensitive to taste loss than others. However, it is unknown whether taste preference is associated with taste alteration in COVID-19 cases.
The aim of this PCR-confirmed case–control study was to investigate the association between taste and smell losses and SARS-CoV-2 infection, and to elucidate whether taste preference affects COVID-19-related taste loss.
Materials and methods
Ethical aspects and setting
This study was conducted at the Bamrasnaradura Infectious Diseases Institute, Department of Health, Ministry of Health, Nonthaburi, Thailand. The study protocol was approved on April 30, 2020 by the Ethics Committee of Bamrasnaradura Infectious Diseases Institute (COA. No. IRB/BIDI S023h/63_ExPD). Written informed consent was obtained from each participant prior to data collection.
Study design and study period
This case–control study was conducted during April to June 2020, which was the period of highest COVID-19 prevalence in Thailand. The case group included persons who tested positive for SARS-CoV-2 by real-time PCR (RT-PCR). The control group comprised persons who tested negative for SARS-CoV-2 by RT-PCR. It is worth noting that during the study period, the SARS-CoV-2 infection in Thailand was mainly the strain found originally in Wuhan, Hubei, China (Manmana et al., 2020).
Participants
Inclusion criteria for participants were Thai nationality, age ≥18 years, able to communicate in Thai, comfortable answering the questionnaire, and had been tested by RT-PCR for SARS-CoV-2. Exclusion criteria included any inability to provide reliable communication; e.g. critical patients on a respirator, unconscious patients, those in a coma, and patients with psychiatric disorders or dementia. All participants signed a written informed consent agreement before data collection. Their identities were protected, in accordance with the Declaration of Helsinki. The case and control groups were matched for age, sex, body mass index (BMI), and systemic disease, with an allocation ratio of two controls per one case.
Sample size and power
The effect size was calculated based on two studies. The incidence of taste and smell changes was 22% in COVID-19 cases in China and Korea (Mao et al., 2020), while alterations in taste and smell were found in 9% of the general population in Korea (Kang et al., 2020). A two-tailed significance level of 0.05 and 90% power were used to calculate the sample size for Fisher’s exact test using the program G-Power version 3.1. Since positivity for SARS-CoV-2 was found in 30% of persons receiving the RT-PCR test at Bamrasnaradura Infectious Diseases Institute, the allocation ratio N2/N1 of 2/1 was applied. The calculation yielded a sample size of 366, including 122 persons for the case group and 244 persons for the control group.
Study procedures
Participants were recruited from the Emerging Infectious Disease Clinic of Bamrasnaradura Infectious Diseases Institute, the central hospital with the highest number of COVID-19 cases in Thailand at that time. The reason for undergoing RT-PCR testing for SARS-CoV-2 was either having respiratory symptoms or having a history of contact with an infected person. All participants were screened by a physician according to the inclusion and exclusion criteria. Then, an epidemiologist who had no duties in patient treatment explained the risks and benefits of the study. All participants voluntarily signed informed consent. Participants completed the questionnaire by themselves during the period they were waiting to be tested for SARS-CoV-2 by RT-PCR method. This timing of data collection could reduce subjective bias, since the participants did not know yet whether they had or did not have the infection. Once the test results were returned, participants with positivity for SARS-CoV-2 were assigned to the case group, while those with a negative result were assigned to the control group.
Outcome assessment
The questionnaire included general information, systemic diseases, medication, history of other respiratory diseases, symptoms, and history of contact with COVID-19 cases. The perception of taste and smell was rated using visual analog scales from 0 (no taste/no smell sense) to 10 (full sense of taste/smell). Ageusia/anosmia were defined as having a taste/smell score of 0, after being suspected of having a SARS-CoV-2 infection. Hypogeusia/hyposmia were defined as having a reduction in taste/smell scores, after being suspected of having a SARS-CoV-2 infection. Preferences for sweet, sour, salty, bitter, umami, fatty, and spicy were studied using TASTE-26, a validated questionnaire (Vittayakasemsont et al., 2019). Each participant answered 21 multiple choice questions to describe their dietary preferences during the past 5 years. Each question was answered on a three-point scale (0–2 points). For example, “How many glasses of sweetened beverages (e.g., soft drinks, coffee, tea, sweetened herbal drink) do you usually drink daily?” is an example of the question for sweet preference. The answer choices for this questions were ≤1 glass/day, 2 glasses per day, ≥3 glasses/day. Three questions were included for the evaluation of each taste preference and the sum of the scores was used to categorize the participant into a strong (score 4–6), moderate (score 2–3), or mild (score 0–1) lover of each taste.
Statistical analyses
Baseline characteristics of the participants in the case and control groups were compared using the Mann–Whitney test for numerical data or the Chi-square test for categorical data. Associations between each variable and SARS-CoV-2 infection were tested statistically by odds ratio (OR), Fisher’s exact test, or Chi-square test. For the multivariate analysis, dummy codes for the outcome variable SARS-CoV-2 infection were defined as 0 = negative, 1 = positive; dummy codes for symptoms were defined as 0 = without the symptom, 1 = with the symptom. Associations between multiple variables and SARS-CoV-2 infection were analyzed using multiple linear regression. Comparisons of taste/smell sense scores between the case and control groups were performed using the Mann–Whitney test. The correlation between taste preferences (ordinal scale) and the change in taste score (delta between before and after scores; numerical scale) was analyzed by Spearman rank correlation analysis. Graphing and statistical analyses were performed using GraphPad Prism version 9.0.0. Power and sample size calculations were performed using G-power version 3.1. Values of P < 0.05 were considered statistically significant.
Results
Characteristics of the study participants
Table 1 shows the baseline characteristics of the participants in the case and control groups. There was no significant difference in age, sex, BMI, or systemic diseases between the groups.
Table 1.
Characteristics of the study participants
| Characteristics | Case group (n = 122) |
Control group (n = 244) |
P-value | ||
|---|---|---|---|---|---|
| Number | % | Number | % | ||
| Sex | 0.55a | ||||
| Female | 64 | 52 | 136 | 56 | |
| Male | 58 | 48 | 108 | 44 | |
| BMI categoryd | 0.50b | ||||
| Obese II | 13 | 10.7 | 28 | 11.5 | |
| Obese I | 24 | 19.7 | 51 | 20.9 | |
| Overweight | 22 | 18.0 | 36 | 14.7 | |
| Normal weight | 52 | 42.6 | 117 | 48.0 | |
| Underweight | 11 | 9.0 | 12 | 4.9 | |
| Systemic diseases | 0.44b | ||||
| None | 100 | 82 | 182 | 75.0 | |
| Allergy, asthma, sinusitis | 19 | 16 | 52 | 21.0 | |
| Otherse | 3 | 2 | 10 | 4.0 | |
| Recent diseases in the past 2 weeks | 0.19b | ||||
| None | 119 | 97.5 | 225 | 92.6 | |
| Streptococcal pharyngitis | 0 | 0 | 7 | 2.9 | |
| Influenza/cold | 3 | 2.5 | 6 | 2.5 | |
| Tonsillitis | 0 | 0 | 2 | 0.8 | |
| Allergy, asthma | 0 | 0 | 3 | 1.2 | |
| Medical treatment | 0.08b | ||||
| None | 109 | 89.3 | 210 | 86.1 | |
| Medicationsf | 6 | 4.92 | 26 | 10.7 | |
| Surgeryg | 7 | 5.74 | 7 | 2.9 | |
| Range | Mean ± SD | Range | Mean ± SD | ||
|---|---|---|---|---|---|
| Age (years) | 20–83 | 39.3 ± 15.12 | 18–90 | 38.1 ± 13.6 | 0.78c |
| BMI (kg/m2) | 14.8–44.08 | 23.3 ± 4.7 | 16.4–45.6 | 23.8 ± 4.8 | 0.54c |
Number, number of participants; %, percentage of the total participants; BMI, body mass index; SD, standard deviation.
Fisher’s exact test.
Chi-square test.
Mann–Whitney test.
Underweight, BMI < 18 kg/m2; normal weight, 18–22.9 kg/m2; overweight, 23–24.9 kg/m2; obese I, 25–29.9 kg/m2; obese II, BMI ≥ 30 kg/m2.
Case group: menopause (n = 2), neurological disorders (n = 1). Control group: menopause (n = 6), thyroid diseases (n = 3), nasopharyngeal cancer (n = 1).
Case group: antihistamines (n = 4), antihypertensives (n = 2). Control group: antihistamines (n = 21), sleeping pills (n = 2), pain killers (n = 2), antibiotics (n = 1).
Case group: rhinoplasty (n = 3), tonsillectomy (n = 2), sinus surgery (n = 1), thyroid surgery (n = 1). Control group: rhinoplasty (n = 4), tonsillectomy (n = 2), brain surgery (n = 1).
Having respiratory symptoms versus contact with an infected person
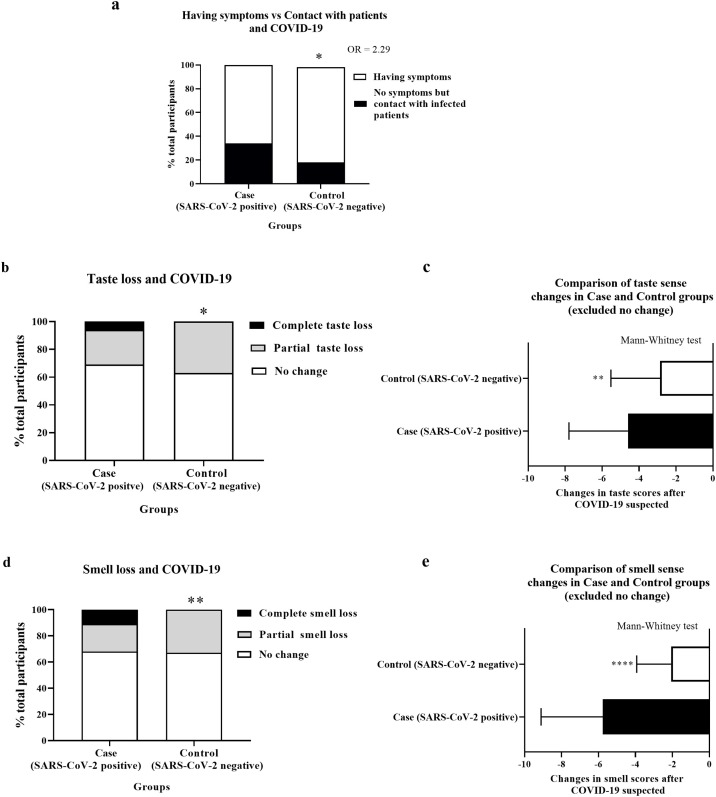
Participants in this study included those either having respiratory symptoms or having been in contact with an infected person. Surprisingly, as shown in Figure 1 a, the control group (SARS-CoV-2-negative) had a higher percentage of participants with symptoms than the case group. Thus, contact with infected persons was more associated with SARS-CoV-2 positivity than having respiratory symptoms (odds ratio (OR) 2.29, 95% confidence interval (CI) 1.199–4.465, P < 0.05).
Figure 1.
Taste and smell alterations and COVID-19.
Image ‘a’: the stacked bars represent the percentage of total participants in the case and control groups who had respiratory symptoms (white) or did not have symptoms but had contact with infected persons (black). P-values derived from Fisher’s exact test; *P < 0.05. OR = odd ratio.
Images ‘b’ and ‘d’: the stacked bars represent the percentage of total participants in the case and control groups who had no change (white), a partial change (grey), or a complete loss (black) of taste (b) or smell (d). P-values derived from the Chi-square test; * P < 0.05, **P < 0.01.
Images ‘c’ and ‘e’: the horizontal bar graphs represents the mean ± standard deviation of the change in self-reported taste (c) and smell scores (e) after COVID-19 was suspected. Negative numbers indicate the magnitude of the reduction in taste or smell perception. P-values derived from the Mann–Whitney test; **P < 0.01, ****P < 0.0001.
Change in taste sense
Hypogeusia and ageusia were found in 25.4% and 5.7% of the case group participants and 37.3% and 0% of the control group participants, respectively (Table 2). Ageusia but not hypogeusia was associated with SARS-CoV-2 positivity with an OR of 14.5 (95% CI 2.157–161.9) (Figure 1b, Table 2). Furthermore, the infected group had a significantly more severe reduction in taste score (−4.6 ± 3.19) when compared to the control group (−2.8 ± 2.66) (P < 0.0001) (Figure 1c). Interestingly, on-and-off taste sense was more frequent in the case group (35%) than in the control group (20%) (P < 0.0001) (Figure 2a).
Table 2.
Association between ageusia or hypogeusia and COVID-19
| Case group (n = 122) |
Control group (n = 244) |
P-valuea | OR | |||
|---|---|---|---|---|---|---|
| Number | % | Number | % | |||
| Complete loss of taste sense (ageusia) | 7 | 5.7 | 0 | 0 | 0.002 | 14.5b |
| Reduced taste sense (hypogeusia) | 31 | 25.4 | 91 | 37.3 | 0.06 | 0.62 |
| No changes | 84 | 68.9 | 153 | 62.7 | ||
Number, number of participants; %, percentage of the total participants; OR, odds ratio.
P-value derived from Fisher’s exact test.
95% confidence interval = 2.157–161.9.
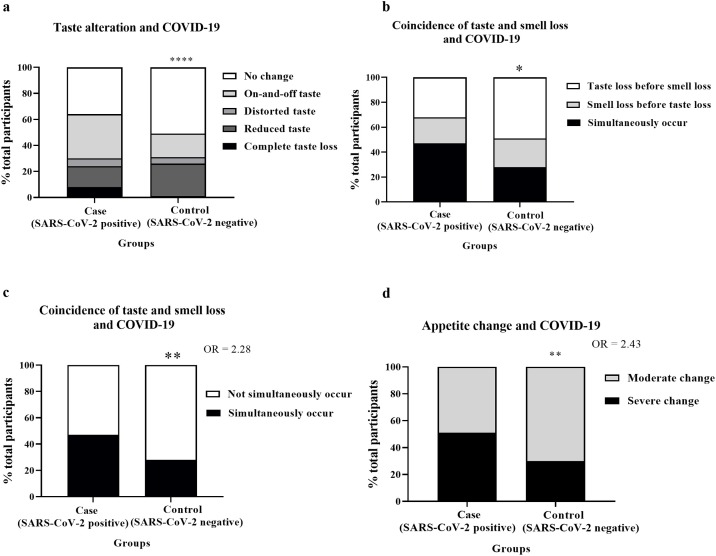
Figure 2.
Coincidence of taste and smell loss and appetite change.
Image ‘a’: The stacked bars represent the percentage of total participants in the case and control groups who had no change (white), on-and-off (light grey), distorted (dark grey), reduced (light black), or complete losses (black) of taste. P-values derived from the Chi-square test; ****P < 0.0001.
Image ‘b’: The stacked bars represent the percentage of participants with taste or smell loss in the case and control groups who had taste loss before smell loss (white), smell loss before taste loss (grey), or simultaneous taste and smell loss (black). P-values derived from the Chi-square test; *P < 0.05.
Image ‘c’: The stacked bars represent the percentage of participants with taste or smell loss in the case and control groups who had simultaneous (black) or not simultaneous (white) loss of taste and smell. P-values derived from Fisher’s exact test; **P < 0.01. OR = odd ratio.
Image ‘d’: The stacked bars represent the percentage of participants with appetite change in the case and control groups who had moderate (grey) or severe (black) loss of appetite. P-values derived from Fisher’s exact test; **P < 0.01. OR = odd ratio.
Change in smell sense
Hyposmia and anosmia were found in 21.3% and 10.7% of the case group participants and in 32.8% and 0% of the control group participants, respectively (Table 3). Anosmia but not hyposmia was associated with SARS-CoV-2 positivity, with an OR of 27.5 (95% CI 4.377–293.6) (Figure 1d, Table 3). Furthermore, the infected group had a significantly more severe reduction in taste score (−5.8 ± 3.34) when compared to the control group (−2.1 ± 1.86) (P < 0.0001) (Figure 1e). Besides the changes in quantity of smell, four of the 122 participants in the case group (3.27%) and 16 of the 244 participants in the control group (6.5%) reported a strange smell on odorless objects.
Table 3.
Association between anosmia or hyposmia and COVID-19
| Case group (n = 122) |
Control group (n = 244) |
P-valuea | OR | |||
|---|---|---|---|---|---|---|
| Number | % | Number | % | |||
| Complete loss of smell sense (anosmia) | 13 | 10.7 | 0 | 0.0 | <0.0001 | 27.5b |
| Reduced smell sense (hyposmia) | 26 | 21.3 | 80 | 32.8 | 0.1 | 0.63 |
| No changes | 83 | 68.0 | 164 | 67.2 | ||
Number, number of participants; %, percentage of the total participants; OR, odds ratio.
P-value derived from Fisher’s exact test.
95% confidence interval = 4.377–293.6.
Association between SARS-CoV-2 positivity and various symptoms
A multivariate analysis was performed to identify associations between SARS-CoV-2 infection and various variables, including the presence of respiratory symptoms, appetite loss, nausea, fever, dry cough, productive cough, difficulty breathing, chest congestion, runny nose, sore throat, fatigue, headache, muscle pain, stomachache, diarrhea, dry mouth, hyposmia, anosmia, hypogeusia, ageusia, and asymptomatic. Only four symptoms had a significant positive correlation with SARS-CoV-2 positivity (Table 4). The standardized regression coefficient estimates (β) of anosmia, ageusia, appetite loss, and fever as predictors for SARS-CoV-2 positivity were 0.39, 0.36, 0.13, and 0.10, respectively.
Table 4.
Multivariate analysis of SARS-CoV-2 infection and various symptoms.
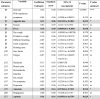 |
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; CI, confidence interval; NS, not statistically significant. Table reports the standardized regression coefficient estimates (β) for the relationship between each symptom and SARS-CoV-2 positivity analyzed by multiple linear regression. Dummy code for outcome (SARS-CoV-2 infection): 0 = negative, 1 = positive. Dummy code for symptoms: 0 = without the symptom, 1 = with the symptom. *P < 0.05, **P < 0.01. Grey highlighted rows indicates symptoms with a significant positive correlation with SARS-CoV-2 positivity. Note: Multiple logistic regression could not be fitted for the data, while multiple linear regression could be fitted well.
Coincidence of taste and smell losses
Most participants in the case group had simultaneous alterations of taste and smell (Figure 2b). Coincidence of taste and smell losses was associated with SARS-CoV-2 positivity (OR 2.28, 95% CI 1.291–4.101, P < 0.01) (Figure 2c).
Appetite
A severe change in appetite was associated with SARS-CoV-2 positivity (OR 2.43, 95% CI 1.337–4.384, P < 0.01) (Figure 2d,).
Taste preference
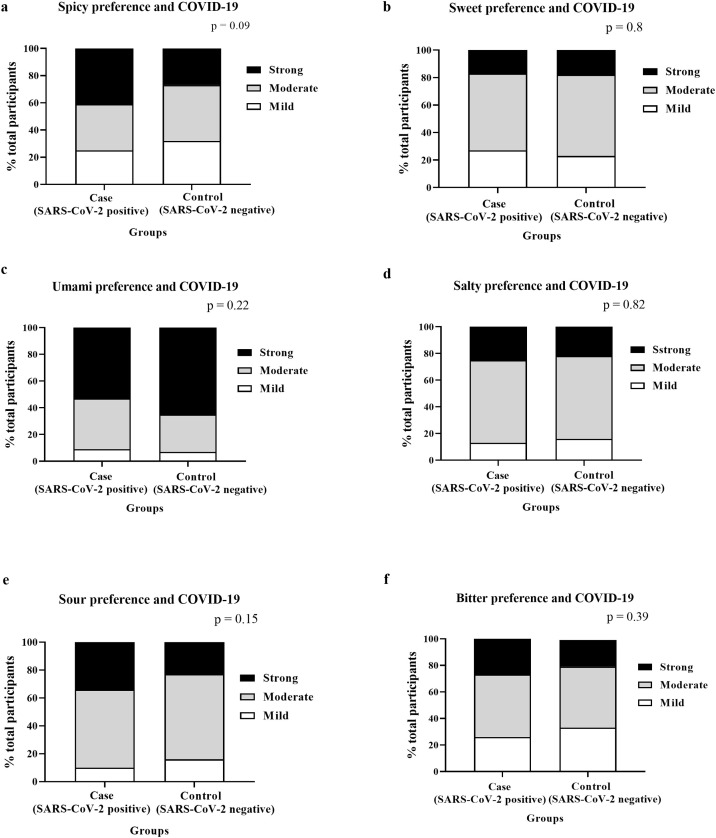
There was no difference in preference for bitter, sour, sweet, spicy, salty, and umami taste between the case and control groups (Figure 3a–f). Likewise, no significant correlations were found between any taste preferences and changes in self-reported taste scores (Table 5).
Figure 3.
Taste preference and COVID-19.
Images ‘a’ to ‘f’: the stacked bars represent the percentage of the total participants in the case and control groups who had a mild (white), moderate (grey), or strong (black) preference for each taste/factor as specified in the figures (a–f). P-values derived from the Chi-square test.
Table 5.
Correlation between taste preference and delta taste perception score
| Preference | Spearman rank correlation coefficient (r) | P-value |
|---|---|---|
| Spicy | −0.017 | 0.85 |
| Umami | 0.0197 | 0.83 |
| Salty | −0.044 | 0.63 |
| Sweet | 0.018 | 0.84 |
| Bitter | 0.0339 | 0.71 |
| Sour | −0.0005 | 0.99 |
The table reports the Spearman rank correlation coefficients for taste preference score (0–6) compared with delta taste score (‘score after’ minus ‘score before’ having symptoms/being exposed to infected persons).
Discussion
Taste and smell losses are emerging symptoms found in COVID-19 patients. However, it is unclear whether these symptoms represent a pathognomonic feature. This matched case–control study found that complete but not partial losses of taste and smell were associated with SARS-CoV-2 positivity. Furthermore, the multivariate analysis of all COVID-19 symptoms suggested that anosmia, ageusia, and appetite loss are the best predictors of SARS-CoV-2 positivity, even better than fever. Consistent with previous studies (La Torre et al., 2020, Carignan et al., 2020), it was found that ageusia and anosmia were strongly associated with SARS-CoV-2 positivity. However, this study is novel in reporting that hypogeusia and hyposmia were not associated with COVID-19. Furthermore, the study findings suggested that coincidence of taste and smell losses along with a severe change in appetite was associated with the infection.
A previous case–control study in Italy reported that ageusia and anosmia were among the symptoms with the highest predictive values for SARS-CoV-2 positivity (La Torre et al., 2020). Likewise, an age-matched case–control study performed in Quebec, Canada also found a strong association between anosmia and ageusia and SARS-CoV-2 positivity (Carignan et al., 2020). Compared to previous studies, the current study was conducted in a larger sample size, with more parameters matched between the groups including age, sex, BMI, and systemic diseases, and more direct data collection instead of an online questionnaire.
The result of no significant association between hypogeusia or hyposmia and SARS-CoV-2 infection was due to the similar incidence of hypogeusia and hyposmia in the control group when compared to the case group. Considering the participants in the control group, the majority of them (75%) had no known systemic diseases (similar to the case group). Interestingly, 80% of participants in the control group had respiratory symptoms as the main reason for getting an RT-PCR test for SARS-CoV-2. Therefore, it is likely that the hypogeusia and hyposmia in the control group could have resulted from other respiratory diseases. In fact, previous studies have shown that allergic rhinitis, sinusitis, and upper respiratory infections such as influenza or even the common cold can also cause olfactory dysfunction and hyposmia (Risso et al., 2020, Malaty and Malaty, 2013, Seiden and Duncan, 2001). Furthermore, 95% of taste disorders are caused by impairment of smell rather than gustatory loss (Malaty and Malaty, 2013, Soter et al., 2008). Moreover, mild cognitive impairment that occurs sub-clinically could also cause reduced taste and smell senses (Steinbach et al., 2010). Therefore, other respiratory diseases besides COVID-19 and subclinical neurological conditions could have been the causes of hypogeusia and hyposmia in the control group. In contrast, the sudden simultaneous loss of taste and smell in healthy persons is rarely seen in other diseases. Cancer treatment, both chemotherapy and head and neck radiotherapy, can also affect taste and smell (Malaty and Malaty, 2013). Nevertheless, none of the current study subjects in the case and control groups had a history of or was undergoing these treatments. Therefore, we propose that the coincidence of anosmia and ageusia could be a pathognomonic feature of COVID-19, especially in healthy persons with no known systemic diseases. A future large-scale study is warranted to confirm this hypothesis.
A previous study showed that taste thresholds and taste preference were correlated with the onset of radiation-induced taste loss (Vittayakasemsont, 2016). In contrast, the current study showed that taste preference had no association with taste loss in COVID-19 patients. This conflicting result suggests different mechanisms of taste loss by radiation and COVID-19. Previous studies have suggested that the mechanism of radiation-induced taste loss is from atrophy of the taste buds rather than nerve injury (Just et al., 2005, Deshpande et al., 2018). In contrast, existing evidence suggests that SARS-CoV-2 may use a trans-synaptic pathway to invade from peripheral neurons such as olfactory and gustatory receptors to the central nervous system, i.e. cranial nerve and brain where taste perception occurs (DosSantos et al., 2020). Thus, the virus may be able to completely and aggressively inhibit the gustatory signals regardless of the original taste threshold levels. It is worth noting that the Thai population generally has high taste thresholds when compared to others such as Japanese (Trachootham et al., 2017). Therefore, future studies in other populations with generally low taste thresholds may be worthwhile. Taste and smell losses affect appetite and food intake (Kershaw and Mattes, 2018). This study showed that SARS-CoV-2 infection was associated with more severe appetite loss when compared to the control group. This finding may help explain the malnutrition in COVID-19 patients reported in previous studies (Thibault et al., 2020, Li et al., 2020). Oral hygiene, especially tongue coat, could reduce the sense of taste (Solemdal et al., 2012). Thus, poor oral hygiene may worsen the taste loss in COVID-19 patients.
Our previous study showed that self-tongue brushing could improve both subjective taste perception and reduce the thresholds of sweet, salty, bitter, and sour taste (Madiloggovit et al., 2016). Therefore, oral hygiene care, especially tongue brushing, should be recommended to COVID-19 patients.
A recent systematic review and meta-analysis reported that the incidence of olfactory taste disorders in Asians was 18%, which was lower than the incidence in Caucasians (Von Bartheld et al. 2020). In the present study, smell disorders (hyposmia and anosmia) and taste disorders (hypogeusia and ageusia) were observed in 32% and 31% of SARS-CoV-2-infected Thai individuals, respectively. This higher incidence than in general Asian populations could be due to the unique Thai culture. Compared to other Asian food, Thai cuisine is considered spicy and has a stronger taste and smell. Our previous study also showed higher taste thresholds in Thai people compared to Japanese people (Trachootham et al., 2017). Due to the high thresholds, Thai people could be at higher risk of sensory loss than other Asians. Further investigations to explore this assumption would be beneficial.
A strength of this study is the matched case–control design in which every participant was confirmed to be SARS-CoV-2-positive or negative by RT-PCR. Furthermore, blinding of the test result at the time of data collection could reduce the subjective bias. The data were collected from participants directly and not by online questionnaire. Nevertheless, a major limitation of this study is that all of the data were self-reported by questionnaire. This was due to a limited access time to the participants. Future studies under better circumstances should include objective measurements of taste, such as filter paper disc or electrogustometry. Changes in appetite may be collected more accurately with diet records of food intake. Previous studies have shown that olfactory taste disorders can appear later than at diagnosis (Spinato et al., 2020, Nakagawara et al., 2020). In this study, the symptoms were evaluated at the time of PCR testing. Thus, the actual prevalence of olfactory taste disorder may be higher. To obtain a more complete picture, future cohort studies with time-course monitoring of taste and smell changes starting from PCR testing until remission are warranted.
In conclusion, the findings of this study suggested that complete but not partial loss of taste and smell were associated with SARS-CoV-2 infection and these could be the best predictors among other symptoms. During the COVID-19 pandemic, healthy persons with sudden simultaneous complete loss of taste and smell should be screened for COVID-19.
Ethics statement
The study protocol was approved on April 30, 2020 by the Ethics Committee of Bamrasnaradura Infectious Diseases Institute (COA. No. IRB/BIDI S023h/63_ExPD).
Data sharing statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
STROBE statement
The authors have read the STROBE 2007 Statement, and the manuscript was prepared and revised according to the STROBE 2007 checklists.
Conflict of interest
D. Trachootham and A. Lam-Ubol received funding for this research from the Dental Innovation Foundation under Royal Patronage, His Majesty the King's Dental Service Unit, Thailand. The other authors have no conflicts of interest to declare.
Author contributions
Trachootham D: obtained the grant, study design, data collection, data analysis, writing; Thongyen S: study design, data collection; Lam-Ubol A: obtained the grant, study design, manuscript editing; Chotechuang N: study design; Pongpirul W: data collection; Prasithsirikul W: study design and data collection supervision.
Acknowledgements
This research was supported by the Dental Innovation Foundation under Royal Patronage, His Majesty the King's Dental Service Unit, Thailand. The authors are grateful to Dr Panithee Thammawijaya, Office of Epidemiology, Department of Disease Control, Ministry of Health for scientific input and co-ordination, Mr Worawut Kulkaew and the research staff of the Dental Innovation Foundation under Royal Patronage for administrative support and co-ordination, Dr Nipa Rojrungvasinkul for statistical suggestions, all of the staff at Bamrasnaradura Infectious Diseases Institute for facilitating the data collection, and all participants for their collaboration in the data gathering.
References
- Bagheri S.H., Asghari A., Farhadi M., Shamshiri A.R., Kabir A., Kamrava S.K. Coincidence of COVID-19 epidemic and olfactory dysfunction outbreak. Med J Islam Repub Iran. 2020;34:62. doi: 10.34171/mijiri.34.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsetto D., Hopkins C., Philips V., Obholzer R., Tirelli G., Polesel J. Self-reported alteration of sense of smell or taste in patients with COVID-19: a systematic review and meta-analysis on 3563 patients. Rhinology. 2020;58 doi: 10.4193/Rhin20.185. 104193/Rhin20.185. [DOI] [PubMed] [Google Scholar]
- Carignan A., Valiquette L., Grenier C., Berchmans M., Nkengurutse D., Marcil-Héguy A. Anosmia and dysgeusia associated with SAR-CoV-2 infection: an age-matched case-control study. Can Med Assoc J. 2020;192(26):E702–E707. doi: 10.1503/cmaj.200869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamoun E., Liu A.A.S., Duizer L.M., Darlington G., Duncan A.M., Haines J. Taste sensitivity and taste preference measures are correlated in healthy young adults. Chem Senses. 2019;44(2):129–134. doi: 10.1093/chemse/bjy082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande T.S., Blanchard P., Wang L., Foote R.L., Zhang X., Frank S.J. Radiation-related alterations of taste function in patients with head and neck cancer: a systemic review. Curr Treat Options Oncol. 2018;19(12):72. doi: 10.1007/s11864-018-0580-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DosSantos M.F., Devalle S., Aran V., Capra D., Roque N.R. Neuromechanisms of SAR-CoV-2: a review. Front Neuroanat. 2020;14:37. doi: 10.3389/fnana.2020.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gane S.B., Kelly C., Hopkins C. Isolated sudden onset anosmia in COVID-19 infection. A novel syndrome. Rhinology. 2020;2020(April) doi: 10.4193/Rhin20.114. [DOI] [PubMed] [Google Scholar]
- Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashmi H.A.S., Asif H.M. Early detection and assessment of COVID-19 (2020) Front Med. 2020;(June) doi: 10.3389/fmed.2020.00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafari M., Seyed Ahadi M., Sahraian M.S. What is the mystery behind anosmia and ageusia in COVID-19? Am J Otolaryngol Head Neck Surg. 2020;3(4):1098. [Google Scholar]
- Kang J.W., Lee Y.C., Han K., Kim S.W., Lee K.H. Epidemiology of anosmia in South Korea: a nationwide population-based study. Sci Rep. 2020;10(1):3717. doi: 10.1038/s41598-020-60678-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kershaw J.C., Mattes R.D. Nutrition and taste and smell dysfunction. World J Otorhinolaryngol Head Neck Surg. 2018;4(1):3–10. doi: 10.1016/j.wjorl.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just T., Pau H.W., Bombor I., Guthoff R.F., Fietkau R., Hummel T. Confocal microscopy of the peripheral gustatory system; comparison between healthy subjects and patients suffering from taste disorders during radiochemotherapy. Laryngoscope. 2005;115(12):2178–2182. doi: 10.1097/01.MLG.0000181502.07160.86. [DOI] [PubMed] [Google Scholar]
- La Torre G., Massetti A.P., Guido A., Fimiani C., Fantini M., Marte M. Anosmia and ageusia as predictive signs of COVID-19 in health care workers in Italy: a prospective case-control study. J Clin Med. 2020;9:2870. doi: 10.3390/jcm9092870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Zhang Y., Gong C., Wang J., Liu B., Shi L. Prevalence of malnutrition and analysis of related factors in elderly patients with COVID-19 in Wuhan, China. Eur J Clin Nutr. 2020 doi: 10.1038/s41430-020-06423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madiloggovit J., Chotechuang N., Trachootham D. Impact of self-tongue brushing on taste perception in Thai older adults: a pilot study. Geriatric Nurs. 2016;37(2):128–136. doi: 10.1016/j.gerinurse.2015.11.004. [DOI] [PubMed] [Google Scholar]
- Malaty J., Malaty I.A.C. Smell and taste disorders in primary care. Am Fam Phys. 2013;88(12):852–859. [PubMed] [Google Scholar]
- Manmana S., Iamsirithaworn S., Uttayamakul S. Coronavirus disease-19 (COVID-19) J Bamrasnaradura Infect Dis Inst. 2020;14(2):124–133. [Google Scholar]
- Mao L., Jin H., Wang M., Hu Y., Chen S., He Q. Neurological manifestations of hospitalized patients with COVID-19 in Wuhan, China. J Am Med Assoc Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawara K., Masaki K., Uwamino Y., Kabata H., Uchida S., Uno S. Acute onset olfactory/taste disorders are associated with a high viral burden in mild or asymptomatic SARS-CoV-2 infections. Int J Infect Dis. 2020;99:19–22. doi: 10.1016/j.ijid.2020.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risso D., Drayna D., Morini G. Alteration, reduction and taste loss: main causes and potential implications on dietary habits. Nutrients. 2020;12:3284. doi: 10.3390/nu12113284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiden A.M., Duncan H.J. The diagnosis of a conductive olfactory loss. Laryngoscope. 2001;111(1):9–14. doi: 10.1097/00005537-200101000-00002. [DOI] [PubMed] [Google Scholar]
- Solemdal K., Sandvik L., Willumsen T., Mowe M., Hummel T. The impact of oral health on taste ability in acutely hospitalized elderly. PLoS One. 2012;7(5):e36557. doi: 10.1371/journal.pone.0036557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soter A., Kim J., Jackman A., Tourbier I., Kaul A., Doty R.L. Accuracy of self-report in detecting taste dysfunction. Laryngoscope. 2008;118(4):611–617. doi: 10.1097/MLG.0b013e318161e53a. [DOI] [PubMed] [Google Scholar]
- Spinato G., Fabbris C., Polesel J. Alterations in smell or taste in mildly symptomatic outpatients with SARS-CoV-2 infection. J Am Med Assoc. 2020;323:2089–2090. doi: 10.1001/jama.2020.6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach S., Hundt W., Vaitl A. Taste in mild cognitive impairment and Alzheimer’s disease. J Neurol. 2010;257(2):238–246. doi: 10.1007/s00415-009-5300-6. [DOI] [PubMed] [Google Scholar]
- Sungnak W., Huang N., Bécavin C., Berg M., Network H.L.B. SAR-CoV-2 entry genes are most highly expressed in nasal goblet and ciliated cells within human airways. ArXiv. 2020 arXiv: 2003.06122v1. [Google Scholar]
- Tanasa I.A., Manciuc C., Carauleanu A., Navolan D.B., Bohiltea R.E., Nemescu D. Anosmia and ageusia associated with coronavirus infection (COVID19) – what is known? Exp Ther Med. 2020;20:2344–2347. doi: 10.3892/etm.2020.8808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault R., Coëffier M., Joly F., Bohé, Schneider S.M., Déchelotte P. How the COVID-19 epidemic is challenging our practice in clinical nutrition-feedback from the field. Eur J Clin Nutr. 2020 doi: 10.1038/s41430-020-00757-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachootham D., Satoh-Kuriwada S., Lam-Ubol A., Promkam C., Chotechuang N., Sasano T. Differences in taste perception and spicy preferences: a Thai-Japanese cross-cultural study. Chem Sense. 2017;43(1):65–74. doi: 10.1093/chemse/bjx071. [DOI] [PubMed] [Google Scholar]
- Vaira L.A., Salzano G., Deiana G., De Riu G. Anosmia and ageusia: common findings in COVID-19 patients. Laryngoscope. 2020;2020(April) doi: 10.1002/lary.28692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittayakasemsont K. Mahidol University; 2016. The association between pre-treatment dietary behavior and taste alteration in Thai head and neck cancer patients who underwent definitive radiotherapy. (Master Thesis) [Google Scholar]
- Vittayakasemsont K., Klaitong C., Phukosi K., Chavasit V., Sinthusek T., Trachootham D. Association between pretreatment dietary preference and weight loss after radiation therapy in head and neck cancer patients: a pilot study. Nutr Cancer. 2019;71(2):230–239. doi: 10.1080/01635581.2019.1578393. [DOI] [PubMed] [Google Scholar]
- Von Bartheld C.S., Hagen M.M., Butowt R. Prevalence of chemosensory dysfunction in COVID-19 patients: a systematic review and meta analysis reveals significant ethnic differences. ACS Chem Neurosci. 2020;11:2944–2961. doi: 10.1021/acschemneuro.0c00460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Coronavirus disease (COVID-19) Situation report (2020a) https://www.who.int/publications/m/item/weekly-epidemiological-update---23-february-2021 Updated on 23 February 2021 [accessed on 23 April 2021].
- World Health Organization. WHO Characterizes COVID-19 as Pandemic-11 March 2020 (2020b). Available online at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen [Accessed 30 December 2020].