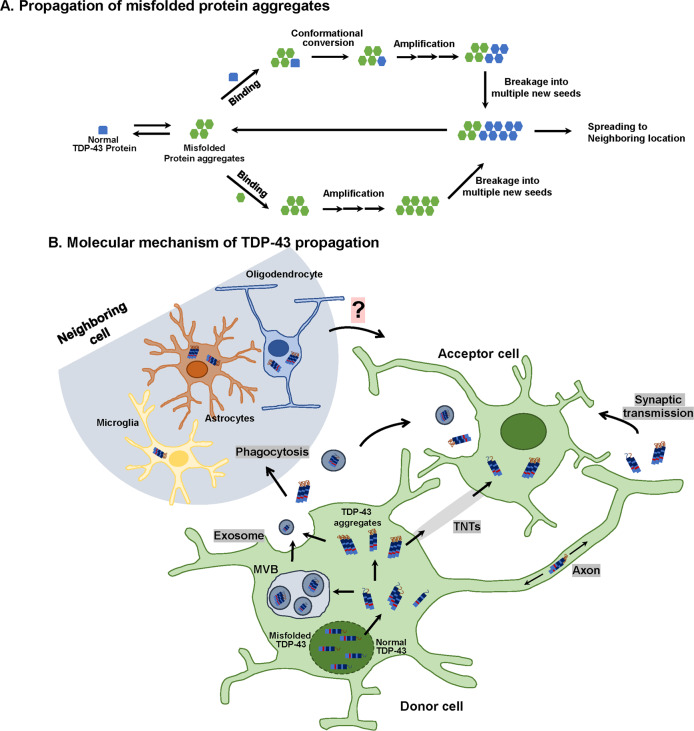
Fig. 2. Schematic overview of misfolded TDP-43 propagation in TDP-43 proteinopathies.
a Proposed mechanism of self-propagation of misfolded TDP-43 in TDP-43 proteinopathies. Misfolded TDP-43 aggregates bind to their normal counterparts and induce the misfolding of bound protein in a template-dependent manner. This process leads to the elongation of misfolded TDP-43 aggregates. Amplification of self-templating amyloid fibrils results from the fragmentation of TDP-43 aggregates, which exposes new ends. b Putative mechanism of cell-to-cell spreading of TDP-43 aggregates. TDP-43 aggregates may propagate via exosomes (release from multivesicular bodies (MVBs)), tunneling nanotubes (TNTs), or synaptic transmission (transport from presynaptic to postsynaptic terminals) from donor cells to acceptor cells. Moreover, glial cells (oligodendrocytes, astrocytes, and microglia) can take up TDP-43 aggregates through phagocytosis, after which misfolded TDP-43 is released from glial cells and transmitted to neurons and neighboring glial cells. The neuron-to-glia or glia-to-neuron transfer of TDP-43 has been observed, but its propagation mechanism is not clear.