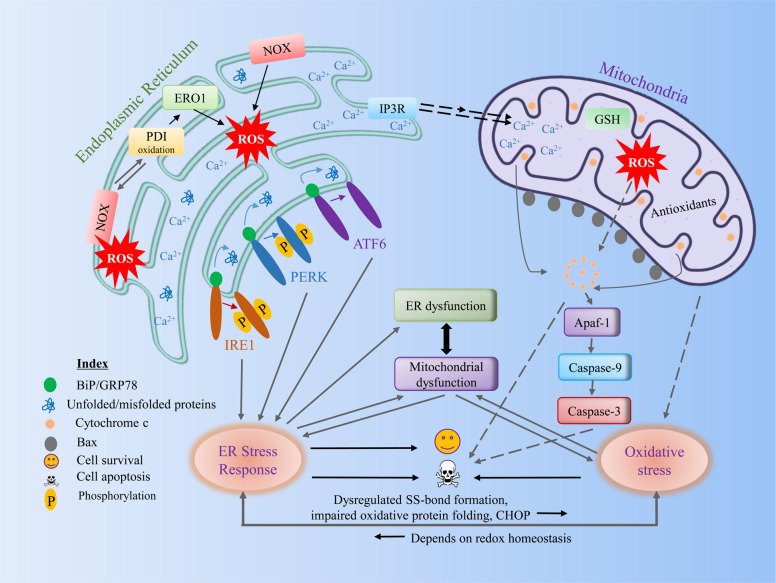
Fig. 2. Cross talk between components of ER stress and the redox signaling pathway.
During various pathological conditions, unfolded or misfolded proteins are increased in the ER. During oxidative protein folding in the ER, ROS are generated during electron transfer between PDI and ERO1α. ROS associated with UPR signaling can activate an antioxidant response, such as Nrf2 or can increase ROS generation by activating ERO1 or NOX. Furthermore, ROS are increased in the ER through the association of PDI with ROS, generating Nox4. Although the major site of calcium is in the ER, under stress conditions, calcium may flow to the mitochondrial outer membrane through calcium release channels, such as IP3R or RyR. Increased calcium overload in mitochondria subsequently increases ROS generation. The increased calcium load and ROS in mitochondria may lead to opening of the mitochondrial permeability transition pore, which may cause the release of proapoptotic factors. High oxidative stress during this condition is critical for inducing mitochondrial dysfunction and vice versa. Overall, we suggest that the ER stress response can induce ER or mitochondrial dysfunction, which may increase oxidative stress by dysregulating disulfide bond formation, impairing oxidative protein folding, or the inducing certain UPR genes (e.g., CHOP) where oxidative stress is reversible, which may depend on redox homeostasis (see the text for more details). ER endoplasmic reticulum, ROS reactive oxygen species, PDI protein disulfide isomerase, ERO1α endoplasmic reticulum oxidoreduction 1α, NOX NADPH oxidase, IP3R inositol 1,4,5-trisphosphate receptors, RyR ryanodine receptors.