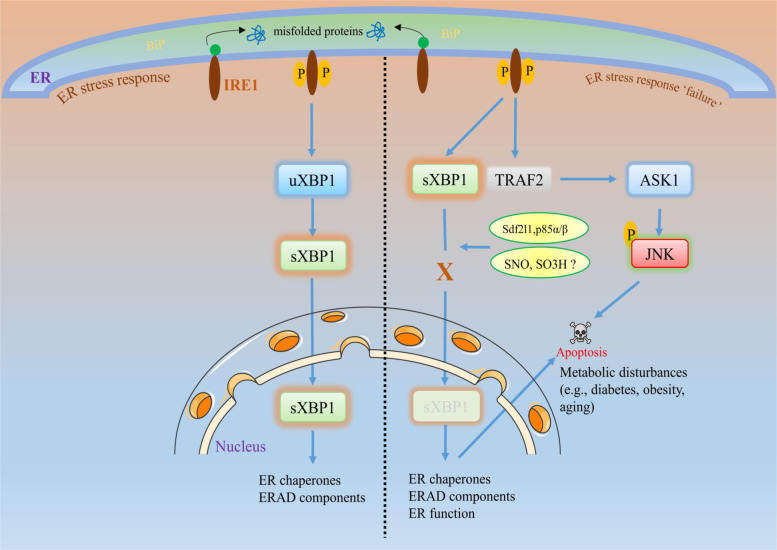
Fig. 3. ER stress response failure and cellular fate.
During acute or short-term ER stress, the cell follows its natural adaptive pathway (as explained in Fig. 1) to maintain cellular homeostasis. However, during prolonged ER stress or under certain conditions, such as aging or metabolic diseases (e.g., obesity or diabetes), the activated UPR sensors may not activate downstream signaling (here, we focus on IRE1 signaling). For example, failure of XBP1s to translocate to the nucleus to activate its target genes leads to decreased activation of XBP1s target genes, such as chaperones or ERAD. This diminished effect is called ER stress response failure, which may trigger apoptotic signaling rather than adaptive responses. Evidence of ER response failure in metabolic diseases suggests that the impaired interaction of XBP1s with the insulin receptor or the regulatory subunits of PI3K p85α and p85β blocks XBP1s translocation to the nucleus. Similarly, excessive reactive oxygen species and/or reactive nitrogen species-induced nitro-oxidative stress production induces sulfonation (SO3H) of IRE1 or SNO of IRE1α, which can decrease IRE1α ribonuclease activity, thereby inhibiting the production of XBP1s88,108,160. This impaired signaling may disrupt the ER chaperones, ERAD, or their functions, which may negatively affect cell survival and trigger apoptosis, leading to the subsequent disease progression.