Abstract
Colony-stimulating factor 1 receptor (CSF1R, also known as c-FMS) is a receptor tyrosine kinase. Macrophage colony-stimulating factor (M-CSF) and IL-34 are ligands of CSF1R. CSF1R-mediated signaling is crucial for the survival, function, proliferation, and differentiation of myeloid lineage cells, including osteoclasts, monocytes/macrophages, microglia, Langerhans cells in the skin, and Paneth cells in the intestine. CSF1R also plays an important role in oocytes and trophoblastic cells in the female reproductive tract and in the maintenance and maturation of neural progenitor cells. Given that CSF1R is expressed in a wide range of myeloid cells, altered CSF1R signaling is implicated in inflammatory, neoplastic, and neurodegenerative diseases. Inhibiting CSF1R signaling through an inhibitory anti-CSF1R antibody or small molecule inhibitors that target the kinase activity of CSF1R has thus been a promising therapeutic strategy for those diseases. In this review, we cover the recent progress in our understanding of the various roles of CSF1R in osteoclasts and other myeloid cells, highlighting the therapeutic applications of CSF1R inhibitors in disease conditions.
Subject terms: Osteoimmunology, Cell signalling, Targeted bone remodelling, Differentiation
Bone disease: Signaling pathway offers promising drug target
Drugs directed at a key signaling receptor involved in breaking down bone tissue could help treat diseases marked by pathological bone loss and destruction. In a review article, Kyung-Hyun Park-Min and colleagues from the Hospital for Special Surgery in New York, USA, discuss the essential roles played by the colony-stimulating factor 1 receptor (CSF1R) protein in the survival, function, proliferation and differentiation of myeloid lineage stem cells in the bone marrow, including bone-resorbing osteoclasts. They explore the links between the CSF1R-mediated signaling pathway and diseases such as cancer and neurodegeneration. The authors largely focus on bone conditions, highlighting mouse studies in which CSF1R-blocking drugs were shown to ameliorate bone loss and inflammatory symptoms in models of arthritis, osteoporosis and metastatic cancer. Clinical trials are ongoing to test therapeutic applications.
Introduction
Osteoclasts are the only bone-resorbing cells and are differentiated from myeloid lineage precursor cells1–5. Osteoclasts play important roles in both homeostatic bone remodeling and pathogenic bone resorption that is associated with inflammatory arthritis, osteoporosis, and bone metastasis in cancer. CSF1R expression is low in immature myeloid precursor cells and increases as the myeloid cells mature6. CSF1R-mediated signaling and receptor activator of NF-κB ligand (RANKL) are essential for osteoclast function, proliferation and differentiation from precursor cells7–11. CSF1/CSF1R signaling induces the expression of receptor activator of NF-κB (RANK), a receptor for RANKL12,13. Upon RANK-RANKL binding, the NF-kB and MAPK signaling pathways are activated to induce MYC and FOS expression, resulting in the induction of metabolic reprogramming and the expression of NFATc1, a master regulator of osteoclastogenesis14. NFATc1 drives the osteoclast differentiation program15,16. Osteoprotegerin (OPG) is a decoy receptor for RANKL and is secreted from stromal cells and osteoblasts17. OPG is a natural inhibitor of RANKL-mediated signaling in osteoclasts by preventing RANK-RANKL interactions and fine-tuning osteoclast differentiation and bone remodeling.
Coordinated regulation between RANKL and costimulatory signals is required for optimal osteoclast differentiation18. Costimulatory signals are mediated by DNAX-associated protein 12 kD (DAP12) and FcεR1 gamma chain (FcRγ), which are adaptor molecules containing immunoreceptor tyrosine-based activation motifs (ITAMs), and are also necessary for osteoclastogenesis19–21. DAP12 and FcRγ pair and bind with cell-surface receptors and transduce ITAM-mediated signaling. DAP12-associated receptors include TREM2, MDL-1, and siglec-15, while FcRγ-associated receptors are OSCAR, PIR-A, and Fcγ receptors (reviewed in ref. 18). DAP12 and FcRγ double-deficient mice exhibit osteopetrosis20,22. Upon ligand binding, two tyrosine residues in the ITAM motif are phosphorylated to recruit Syk kinase and activate downstream signaling pathways, which activate PLCγ and calcium signaling to promote RANKL-induced NFATc1 expression. DAP12 is also phosphorylated by CSF1R activation23, and crossregulation between DAP12-mediated signaling and CSF1R-mediated signaling in osteoclasts has also been reported24.
The essential role of the CSF1/CSF1R axis in osteoclasts has been well established. CSF1R is a type III receptor tyrosine kinase (RTK) that is involved in the proliferation, differentiation, survival, motility, and function of myeloid cells and in promoting disease progression in various conditions ranging from inflammation to cancer7,25,26. An autosomal recessive inactivating mutation of the CSF1 gene in op/op mice causes osteopetrosis and other developmental defects that are associated with reduced numbers of osteoclasts and macrophages27. The administration of CSF1 to op/op mice rescues the defects in osteoclasts and osteopetrosis28. CSF1R-deficient mice largely recapitulate the phenotype of op/op mice and exhibit abnormal skeletal, neural, and glandular development29. CSF1R-deficient mice show reduced macrophage and osteoclast numbers and reduced matrix remodeling due to diminished cellular motility and adhesion26,30. However, the severity of the osteopetrotic phenotype and systemic depletion of macrophages are much higher in CSF1R-deficient mice than in op/op mice, while the numbers of Langerhans cells and microglia in CSF1R-deficient mice are comparable to those of op/op mice. In op/op mice, hematopoietic deficiencies have been shown to be resolved with age30. This discrepancy was explained later by the discovery of interleukin-34 (IL-34), another ligand of CSF1R31. Currently, CSF1 and IL-34 are the two known ligands of CSF1R. Both ligands induce osteoclast differentiation11,32.
CSF1R also plays an important role in the differentiation of osteoclasts during the developmental period. During embryonic development, macrophage-like cells are produced first in the yolk sac and then appear in the liver33. Csf1r mRNA has been detected in the ectoplacental cone early after implantation and in phagocytic cells isolated from the yolk sac34. Fate mapping using Csf1r-Mer-iCre-Mer;Rosa26TdTomato mice shows that CSF1R-positive yolk sac macrophages are differentiated from early erythromyeloid progenitors (EMPs) and give rise to neonatal osteoclasts35. Conversely, CX3CR1+ yolk-sac macrophages provide long-lasting osteoclast precursors for postnatal bone remodeling in both physiological and pathological conditions. Osteoclasts originating from EMP-lineage cells are found in fetal ossification centers and are involved in normal bone development and tooth eruption. Postnatal maintenance of osteoclasts comes from the fusion process between long-lived syncytia and hematopoietic stem cell (HSC)-derived circulating cells that express CX3CR136. In bone fracture and during homeostasis, circulating CX3CR1+ osteoclast precursor cells migrate to the bone and become CX3CR1−TRAP+ osteoclasts37. Therefore, CSF1R-mediated signals are important for both osteoclast precursor cells and osteoclasts.
Mutations in CSF1R that cause the expression of a mutant receptor or inactivation of one Csf1r allele have been identified in rare neurodegenerative disorders: adult-onset leukodystrophy with axonal spheroids and pigmented glia (ALSP) (also known as hereditary diffuse leukoencephalopathy with axonal spheroids (HDLS)), pigmented orthochromatic leukodystrophy (POLD), and pediatric-onset leukoencephalopathy38–41. Inactivation of one allele of Csf1r is sufficient to cause ALSP in a mouse model42 with some differences in neuropathological findings43. In addition, circulating slan+CD14+CD16+ monocytes that express high levels of CSF1R are depleted in patients with HDLS44. In inflammatory brain disorders, circulating monocytes might enter and repopulate the brain, although microglia originate from the yolk sac45. While HDLS patients with monoallelic CSF1R mutations do not exhibit osteopetrosis or bone abnormalities38, biallelic CSF1R mutations cause skeletal disorders and osteosclerosis41,46. Patient studies have established the important role of CSF1R in the regulation of not only microglia in the brain but also CSF1R-sensitive blood myeloid cells. Analyses of bone cells and bone phenotypes in patients with CSF1R mutations need to be further conducted to establish the link between osteoclasts and CSF1R-mediated signaling in humans.
In this review, we describe the basic aspects of CSF1R, the function of CSF1R in osteoclasts, and the effect of pharmacological inhibition of CSF1R on disease progression in preclinical and clinical settings.
The structure of CSF1R
The CSF1R gene
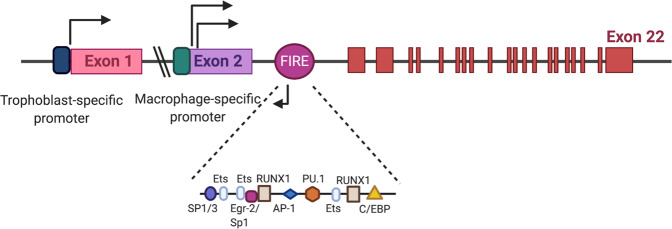
Only myeloid cells express Csf1r mRNA (Fig. 1: the murine Csf1r gene). The Csf1r gene is located on human chromosome 5 (5q32)47 and in a syntonic region on mouse chromosome 18 (18D)48. The Csf1r gene consists of 21 introns and 22 exons. The expression of the Csf1r gene is mediated by two alternative promoters and occurs in a tissue-specific manner. The first exon of Csf1r is transcribed only in trophoblasts, and the second exon of Csf1r is transcribed in macrophages. Deletion of the trophoblast-specific promoter regions in the Csf1r-EGFP transgenic line also abolishes the expression of EGFP in osteoclasts49. The transcriptional activation of Csf1r involves many transcription factors, including Ets (the E26 transformation-specific family of transcription factors), PU.1, ATF, C/EBP, RUX, AP-1, IRF, STAT, KLF, REL, and FUS/TLS50. The Csf1r promoter is filled with multiple PU.1/Ets binding sites around the transcription start site. However, the proximal CSF1R promoter lacks a TATA box and other classic promoter elements. It has been suggested that a loose repeat of CAG or CAA immediately adjacent to the dominant start site bound by Ewing sarcoma (EWS) and FUS/TLS, which are two TATA-associated factors, might substitute for the TATA-binding protein in macrophages51.
Fig. 1. Genomic structure of the mouse Csf1r locus.
Both human and mouse Csf1r genes consist of 22 exons and 21 introns (upper panel). Exon 1 is only expressed in trophoblasts through activation of the trophoblast-specific promoter. The human trophoblast-specific promoter is located 20 kb upstream of exon 1. Csf1r transcription in macrophages starts from the promoter that is upstream of exon 2. Neither promoter has a TATA box. The Fms-intronic regulatory element (FIRE) is a highly conserved regulatory element that produces antisense transcripts to overcome the unknown repressive elements in intron 2. Several transcription factors known to bind to the FIRE have been characterized (lower panel).
The expression of the Csf1r gene is regulated by two highly conserved regions: the promoter upstream of exon 2 and the fms intronic regulatory element (FIRE). Yue et al. show that in macrophages, a 3.5 kb exon 2 promoter facilitates the maximal expression of Csf1r and further suggest that the 0.3 kb promoter is as active as the 3.5 kb promoter52. Moreover, the FIRE is a 250-bp region in intron 253. The FIRE is an important regulatory element of the CSF1R gene in macrophages and controls transcript elongation during macrophage-specific transcription of CSF1R54. The FIRE contains the consensus binding sites for many transcription factors (Fig. 1)55. The FIRE also encodes antisense transcripts that start from two antisense transcription start sites6. Inverting the orientation of the FIRE diminishes its enhancer activity in macrophages54, suggesting that the FIRE is an orientation-specific transcriptional enhancer element. An antisense transcript encoded by the FIRE may contribute to its ability to overcome repression by uncharacterized repressive elements within intron 2. During the differentiation of macrophages from immature precursor cells, the recruitment of transcription factors and chromatin remodeling occur first in the proximal Csf1r promoter and then in the FIRE, allowing only differentiated macrophages to express higher levels of Csf1r. However, a recent study shows that the genomic deletion of the FIRE in mice selectively impacts CSF1R expression and demonstrates the functional importance of the FIRE only in specific macrophage populations56. Ablation of the FIRE in mice depletes embryonic macrophages in embryo and tissue macrophages, including microglia in the brain and resident macrophages in the skin, kidney, heart, and peritoneum56. In contrast to CSF1R-deficient mice, FIRE-deficient mice are healthy and fertile without growth, neurological, or developmental abnormalities such as osteopetrosis and failure of tooth eruption, suggesting that the FIRE is not required for Csf1r expression in all types of myeloid cells.
The CSF1R protein
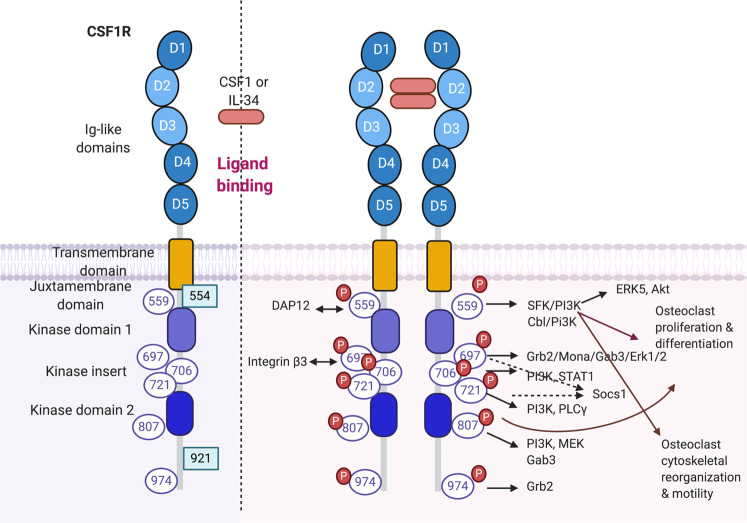
The structures of human CSF1R and mouse CSF1R are highly conserved. CSF1R is divided into two parts: the extracellular domain and the intracellular cytoplasmic domain (Fig. 2)57,58. The extracellular domain contains immunoglobulin (Ig)-like domains to which ligands bind, a linker region, and a single-pass transmembrane helix. Three N-terminal Ig domains (D1–D3) contribute to ligand recognition, while the next two Ig domains (D4–D5) are involved in stabilizing the ligand-receptor complex. The cytoplasmic domain consists of two-kinase domains, a kinase insert, a juxtamembrane domain, and a carboxyterminal tail. CSF1R also undergoes posttranslational modifications such as phosphorylation and glycosylation. In the absence of ligands, CSF1R is in an inactive autoinhibitory state. Upon ligand binding, the juxtamembrane domain moves from the autoinhibitory position, and CSF1R shifts to an activated, extended conformation59.
Fig. 2. Structure of CSF1R protein.
The left panel shows the structure of CSF1R. The extracellular regions have five Ig-like domains; D2 and D3 are ligand binding domains. The intracellular domains consist of the transmembrane domain, the juxtamembrane domain, two-kinase domains, a kinase insert, and cytoplasmic domains. CSF1R is a receptor tyrosine kinase and has 6 tyrosine residues that are phosphorylated upon ligand binding (purple circle). Green square: phosphorylated tyrosine in the v-FMS oncogenic receptor. Ligand engagement of CSF1R induces dimerization and phosphorylation of tyrosine residues. Phosphorylated tyrosines serve as docking sites for Src homology domain 2-containing signaling molecules to promote osteoclast differentiation, proliferation, cytoskeletal reorganization, and motility.
CSF1R-expressing cells have been identified using CSF1R-EGFP transgenic mice60,61. The regulatory elements of the murine Csf1r locus, including 150 bp of the distal promoter, have been used to generate CSF1R-EGFP mice. EGFP expression is detected in placental trophoblasts from the earliest stage of implantation at embryonic day (ED) 7.5, as well as in various stages of development58. During embryonic development, EGFP expression is also detected in cells from the yolk sac at ED 8.5–9, in the head of embryo at ED 9.5, in the dorsal midline at ED 10.5, in the skin at ED12.5, and in F4/80-positive cells at ED 11–12. Csf1r-mApple transgenic mice also exhibit a similar pattern of expression as Csf1r-EGFP mice62. CSF1R expression begins in the early embryonic developmental stage in oocytes and in preimplantation embryos63,64. CSF1R protein expression is restricted to myeloid cells and is much higher in tissue resident macrophages than in blood monocytes.
CSF1R signal transduction pathways
CSF1R signal transduction pathways play key roles in the survival, differentiation, and functions of myeloid cells and have been extensively studied in macrophages, osteoclasts, and microglia (reviewed in ref. 58).
CSF1R signaling pathways
Ligand binding leads to rapid dimerization and autophosphorylation of CSF1R (Fig. 2). Six tyrosine residues in the CSF1R cytoplasmic domain (Y559, Y697, Y706, Y721, Y807, and Y974) and two tyrosine residues in an oncogenic form of Csf1r (Y544 and Y921) have been described as being phosphorylated65,66. Most of these phosphorylated tyrosines serve as docking sites for adaptor proteins containing the Src homology 2 (SH2) domain, and these adaptor proteins further relay downstream signaling events. The function of each tyrosine residue is determined based on mutagenesis experiments. Upon ligand binding, Tyr559 in mouse CSF1R or Tyr561 in human CSF1R is the first phosphorylated tyrosine, providing a docking site for Src family kinase (SFK)/Cbl and regulating ERK5 and Akt activity. Cbl is an E3 ubiquitin kinase67, and activation of SFK/Cbl leads to the multiubiquitination of CSF1R and receptor internalization and degradation68. Phosphorylated Cbl also recruits and stabilizes multiprotein complexes. Tyr559 maintains CSF1R inactivity in the absence of ligands66. Ligand-induced phosphorylation of Tyr559 releases autoinhibition and permits full activation of CSF1R in macrophages69. Tyr559 plays a critical role in CSF1R activation, cell proliferation, intracellular signaling, and cell death of macrophages relative to those of Tyr697 and Tyr80770. In addition, SFK connects to the DAP12 adaptor protein, which is also phosphorylated by CSF1R activation58. DAP12 is required for CSF1-induced phosphorylation and stabilization of β-catenin23. DAP12 deficiency impairs CSF1R-mediated macrophage proliferation and survival. Tyr697, Tyr706, and Tyr721 are located in the kinase insert domain. Tyr697 is a docking site for growth factor receptor-bound protein-2 (Grb2)71. Grb2 is involved in CSF1R-mediated ERK activation through the nucleotide exchange factor Sos72. Mona is an adaptor protein that is induced by CSF1. Mona has one SH2 domain and two SH3 domains and binds to Grb2 via Tyr697 in CSF1R73. After the first wave of tyrosine phosphorylation, including the transient formation of the CSF1R/Grb2/Sos complex, the second wave of tyrosine phosphorylation is accompanied by serine phosphorylation and cbl-dependent CSF1R ubiquitination74. Tyr706 is associated with phosphatidylinositol 3 (PI3)-kinase75 and mediates CSF1-induced STAT1 activation76. Tyr706 also suppresses the expression of CD11b in macrophages77. Tyr721 is the binding site for PI3K and PLCγ, which leads to macrophage differentiation78. Tyr807 and Tyr921 are in the carboxyterminal tail of CSF1R. Tyr807 is conserved throughout all protein tyrosine kinases and is required for macrophage differentiation79. The v-FMS oncogene of feline sarcoma virus encodes an oncogenic FMS protein that differs from CSF1R in only seven amino acids and in the C-terminal region. Two additional tyrosines in v-FMS are phosphorylated. Tyr543/544 is located in the juxtamembrane domain and is associated with the p55 polypeptide80. In addition to Tyr696, Tyr921 in the vFMS oncogene also serves as the second docking site for Grb265.
In addition to ligand-induced tyrosine phosphorylation, the ligand-CSF1R complex is internalized by endocytosis, which quickly terminates CSF1R signaling and leads to the degradation of CSF1R and CSF181,82. In addition, Huynh et al. reported that internalized CSF1R mediates sustained ERK1/2 and Akt signaling83. Early CSF1R signaling (~2 h) also induces increased protein synthesis and regulates macrophage protein turnover84. In addition, ligand-induced ectodomain shedding may regulate CSF1R activity. CSF1R signaling pathways also regulate macrophage morphology, adhesion, and motility85. Ablation of CSF1R leads to macrophage rounding by increasing the level of protein tyrosine phosphatase phi/paxillin/Pky2 complexes86.
CSF1R signaling in osteoclasts
CSF1R-mediated signaling plays important roles in the differentiation of osteoclast precursor cells and mature osteoclasts. Csf1r-deficient mice or op/op mice have a severe osteoclast deficiency and weak long bones that show a disorganized matrix, reduced mineralization, and abnormal osteoblasts27,87. During in vitro osteoclastogenesis, CSF1R-mediated signaling induces RANK expression in osteoclast precursor cells. The role of tyrosine phosphorylation of CSF1R in osteoclasts has been investigated using Epo receptor/CSF1R chimeras88. In the experiment, each tyrosine in the Epo/CSF1R receptor is mutated to phenylalanine. When stimulated with Epo and RANKL, cells expressing the Y559F mutant receptor fail to differentiate into osteoclasts, while those expressing either Y559F or Y807F fail to resorb bone. This study shows that Tyr559 and Tyr807 in CSF1R are essential for osteoclast proliferation and differentiation, whereas Tyr697, Tyr706, and Tyr721 exert no effects on osteoclasts88. Cbl is able to bind to phosphorylated Tyr559. In osteoclasts, Cbl-PI3K does not affect CSF1R-mediated proliferation and differentiation of precursors but is required for survival and actin reorganization in mature osteoclasts89.
In addition to the differentiation and proliferation of osteoclasts, CSF1R-mediated signaling stimulates motility and regulates cytoskeletal reorganization in a c-Src-dependent manner via Tyr55990. Crosstalk between CSF1R and other important molecules in osteoclasts, including integrin β3 and DAP12, regulates cytoskeletal reorganization and adhesion of mature osteoclasts. Integrin β3 is induced by RANKL stimulation and binds to extracellular matrix proteins such as vitronectin, osteopontin, and bone sialoprotein91. Integrin β3-deficient mice have dysfunctional osteoclasts and develop an osteosclerotic phenotype91–93. However, in vitro osteoclastogenesis of integrin β3-deficient cells is blunted, and enhanced CSF1/CSF1R-mediated signaling rescues the defective osteoclasts in integrin β3-deficient mice94. High-dose CSF1 treatment promotes prolonged ERK activation and c-FOS expression in integrin β3-deficient mice mediated by Tyr697 in CSF1R. DAP12 is an adaptor molecule containing an immunoreceptor tyrosine-based activation motif (ITAM) and is necessary for osteoclastogenesis19–21. Activation of DAP12-associated receptors induces the phosphorylation of ITAM by Src family kinases and recruits Syk or Zap70 tyrosine kinases, which regulate cytoskeletal remodeling in osteoclasts. DAP12 is activated by CSF1R-mediated signaling and is involved in CSF1R-mediated cytoskeletal remodeling in osteoclasts24. Tyr559 is necessary for the activation of DAP12/syk-mediated signaling. A high dose of CSF1 also partially rescues the defects in osteoclastogenesis in DAP12-deficient cells21. In addition, it has been shown that pretreatment with inflammatory signals such as LPS and IL-1 prior to RANKL stimulation suppresses osteoclastogenesis in part by inducing ectodomain shedding and CSF1R degradation95,96. In both physiological and pathological conditions, CSF1R-mediated signaling contributes to osteoclast differentiation and activity. However, the downstream signaling networks of CSF1R in osteoclasts are an area of research that needs further investigation.
CSF1R ligands and their differential functions in CSF1R signaling
CSF1R binds to two different ligands: CSF1 and interleukin-34 (IL-34)31. Although these ligands share the same receptor, there are structural, functional, and spatial differences between CSF1 and IL-34. IL‐34 has no apparent consensus structural domain or motif and shares no sequence similarity with CSF197. The CSF1/CSF1R complex and the IL-34/CSF1R complex also do not share structural similarities97. The CSF1/CSF1R complex has hydrophilic interactions, while the IL‐34/CSF1R complex contains a large number of hydrophobic regions. While CSF1 is detectable in circulation, IL-34 is not circulated in blood. Although CSF1R is only receptor for CSF1, IL-34 has been shown to bind to the extracellular domains of CSF‐1R, receptor‐type protein‐tyrosine phosphatase‐zeta (PTP‐ζ), and the chondroitin sulfate chains of syndecan‐198,99. Although IL-34 and CSF1 have equivalent ability to induce macrophage differentiation with comparable kinetics, CSF1 and IL-34 have different capability for macrophage polarization. IL-34-derived M1 and M2 macrophages have significantly higher secretion of IL-10 and CCL17, respectively, than their CSF1-derived counterparts100. Furthermore, macrophages differentiated from human peripheral blood monocytes with IL-34 exhibit greater resistance to HIV-1 infection than those derived from CSF1 due to increased expression of pertinent restriction factor genes101. IL-34 has also been demonstrated to repolarize rat M1 Kupffer cells (KCs) in the liver to the M2 phenotype by activating the PI3K/protein kinase B/mammalian target of rapamycin (mTOR) pathway and could be used therapeutically to reduce acute rejection during liver transplantation102. Each of these ligands might play a different role in osteoclasts and other myeloid cells, which are discussed below.
CSF1
CSF1 (also known as macrophage colony-stimulating factor (M-CSF)) is a ligand for CSF1R and a growth factor that regulates the survival, proliferation, and differentiation of cells of hematopoietic lineages (reviewed in refs. 103,104). The CSF1 gene is located at chromosome 1 p21-p13 in humans and at chromosome 3, 51 cM in mice105. CSF1 exists as a membrane-bound protein, a secreted proteoglycan, and a secreted glycoprotein. Furthermore, CSF1 has several isoforms. Short, membrane-bound CSF1 is generated from 1.6 and 3.1 kb transcripts, and 2.6, 3.7, and 4 kb transcripts generate the secreted forms of M-CSF. CSF1 also forms a homodimer106. CSF1 is widely expressed in many cell types, including osteoblasts, stromal cells, fibroblasts, epithelial cells, and several metastatic tumor cells107–110. CSF1 is secreted as a glycoprotein or proteoglycan111,112 or is expressed as a cell-surface protein on the cell membrane113. It has been established that cell-surface CSF1 and secreted CSF1 differentially regulate osteoclastic bone resorption. CSF1 alone can correct osteoclast deficiency in CSF1-deficient op/op mice114. Although it has been shown that membrane CSF1 is important for osteoblast-mediated osteoclastogenesis in the bone microenvironment115–118, transgenic op/op mice expressing membrane-spanning cell-surface CSF1 cannot fully recover from osteopetrosis and hematologic abnormalities in the blood and bone marrow and show delayed trabecular bone resorption119.
Systemic injection of CSF1 exacerbates the symptoms of collagen-induced arthritis (CIA) and acute-induced arthritis, suggesting the involvement of CSF1 in inflammatory arthritis by increasing myeloid cells120,121. In an inflammatory osteolysis model, TNFα induces CSF1 production in stromal cells, and CSF1 contributes to increased osteoclastogenesis122. In several pathological bone diseases, including osteoporosis and inflammatory arthritis, a correlation has been observed between an increased number of osteoclasts and elevated levels of CSF1123–126. In addition to enhancing inflammatory responses127, CSF1 also plays a role in pain development associated with inflammatory arthritis128,129. However, the exact underlying mechanism of CSF1 in inflammatory arthritis is not completely understood. Estrogen suppresses the mRNA and protein expression of CSF1 by controlling Egr-1 and Sp-1130,131. In an estrogen-deficient state, increased Sp-1 binds to the Csf1 gene and enhances CSF1 expression. In addition, elevated serum CSF1 has been detected in patients with breast cancer and have been correlated with an adverse prognosis132,133. As such, CSF1 is considered a potential prognostic marker for patient survival and disease recurrence134.
IL-34
IL-34 is a cytokine that binds to CSF1R and is synthesized as a secreted glycoprotein31,135. Similar to CSF1, IL-34 activates downstream signaling pathways and regulates major cellular functions, including proliferation, differentiation, survival, metabolism, cellular adhesion, migration, and cytokine/chemokine expression. IL‐34 exists in all vertebrates, including fish, amphibians, birds, and mammals and exhibits high conservation among species. Structurally, IL‐34 belongs to the short‐chain helical hematopoietic cytokine family but shows no apparent consensus structural domains, motifs, or sequence homology with other cytokines. The IL-34 gene is located in humans on chromosome 16q22.1 and in mice on chromosome 8E131. In the steady state, IL‐34 contributes to the development and maintenance of specific myeloid cell subsets in a tissue‐specific manner: Langerhans cells in the skin and microglia in the brain63,136,137. IL-34 has important roles in the differentiation of Langerhans cells (LCs) and microglia in the gray matter138,139. IL-34 is also present at the fetal-maternal interface and plays a key role in the polarization of macrophages to a decidual phenotype140. Furthermore, IL-34 is essential for the movement of yolk sac macrophages to the embryonic brain and the earliest seeding of the brain by microglia141. Brain-derived IL-34 is responsible for the migration of microglial precursors to the proximal brain region142. Finally, IL-34 secreted from the follicular dendritic cell line FL-Y was found to induce the differentiation of a new type of monocytic cell called follicular dendritic cell-induced monocytic cells by associating with the molecular chaperone 78-kDa glucose regulated protein143.
In addition to its function in mammals, IL-34 is required in aquatic organisms as well. IL-34 homologs from mudskippers (Boleophthalmus pectinirostris) play key roles in the differentiation of mudskipper monocytes and macrophages into proinflammatory phenotypes144. In zebrafish, ectopic expression of IL-34 stimulates the in vivo enrichment of macrophages in the liver145. In one frog species (Xenopus laevis), IL-34-derived macrophages have a higher expression of pattern recognition receptor (PRR) genes related to viral and bacterial pathogen-associated molecular pattern (PAMP) recognition than their CSF1-driven counterparts146.
IL-34 is a novel regulator of osteoclastogenesis and plays a key role in pathological bone destruction by driving the onset and development of inflammatory arthritis. IL-34 induces osteoclast formation in human CD14+ monocytes147. IL-34 promotes the proliferation and differentiation of murine bone marrow-derived macrophages (BMMs) into osteoclasts in lieu of CSF132. Furthermore, IL-34 alone provides a sufficient signal for BMM survival, and in combination with RANKL, it increases the expression of p-STAT3. Treatment with AG490, an inhibitor of JAK2/STAT3 signaling, reduced both IL-34- and RANKL-driven osteoclastogenesis and increased Smad7 expression, while the inhibitor had a negligible effect on osteoclastogenesis induced by M-CSF and RANKL148. These results suggest that IL-34 may regulate osteoclastogenesis in part by increasing p-STAT3 and decreasing Smad7. Furthermore, IL-34 is expressed in the mouse multiple myeloma (MM) cell line MOPC315. BM supports osteoclastogenesis in vitro and accelerates MM-induced osteolysis in vivo.
In pathological conditions, changes in IL‐34 expression are correlated with disease progression, severity, and chronicity149. Due to its osteoclastogenic effect, IL-34 is continuously being investigated as a potential diagnostic marker for inflammatory bone and joint diseases such as rheumatoid arthritis (RA). Plasma levels of IL-34 and RANKL in patients with RA are significantly higher than those of healthy controls. Furthermore, there is a significant correlation between IL-34 and bone erosion, as measured by ultrasound150. Similarly, serum levels of IL-34 in patients with psoriatic arthritis are significantly higher than those in patients with psoriasis alone or healthy controls151. Mechanistically, it has been shown that IL-34 could promote rheumatoid fibroblast-like synoviocytes (FLS) to produce IL-6, which increased the level of Th17 and further advanced RA152. Furthermore, IL-34 levels in gingival crevicular fluid (GCF) of patients with either chronic or aggressive periodontitis were higher than those of healthy controls153. Finally, IL-34 levels in the serum and synovial fluid of patients with osteoarthritis (OA) correlated with the radiographic and symptomatic severity of OA154.
In addition, IL-34 shows promise as a diagnostic marker for diverse inflammatory conditions, including liver fibrosis, atherosclerosis, Hashimoto’s thyroiditis, and lupus nephritis. The serum level of IL-34 is related to inflammatory activity in the liver and is highly sensitive to severe liver fibrosis in patients with chronic hepatitis B virus infection155. IL-34 treatment increases the formation of foam cells by upregulating CD36 expression through the p38-MAPK signaling pathway in bone marrow-derived macrophages, suggesting its role in promoting the development of atherosclerosis156. In patients with Hashimoto’s thyroiditis, the serum level of IL-34 was significantly less than that of healthy controls and could be evaluated to measure thyrocyte damage157. Moreover, patients with lupus nephritis (LN) have higher levels of IL-34 in the serum than healthy controls158. A study using MRL-Faslpr mice, a mouse model of lupus, revealed that mice lacking IL-34 exhibit decreased nephritic symptoms. In mice, IL-34 causes intrarenal macrophages to accumulate by inducing monocyte proliferation in the bone marrow and stimulates tubular epithelial cells to undergo apoptosis159. A study using human mesangial cells (HMCs) discovers that IL-34 is highly expressed in the HMCs of LN patients and is suppressed by treatment with DDK1, an inhibitor of the Wnt pathway160.
Diseases and therapeutic applications of CSF1R inhibition
CSF1R-mediated signaling plays an important role in many diseases, including inflammatory arthritis, neurodegenerative diseases, Alzheimer’s disease, cancer, atherosclerosis, lung fibrosis, diabetes, multiple sclerosis, and systemic lupus erythematous161. Myeloid cells have been found to promote disease progression, and either blocking the differentiation or recruitment of myeloid cells is considered a potential strategy to attenuate disease conditions. Various approaches targeting either CSF1R or its ligands are currently in clinical development (Table 1). In addition, CSF1R inhibition has been relatively well tolerated and demonstrates good specificity. CSF1R targeting has shown beneficial effects in models of cancer metastasis and inflammatory diseases although deleterious effects have been also observed in colitis162 and skeletal muscle regeneration163. Thus, the use of CSF1R inhibitors or antibodies might be a promising strategy for treating myeloid cell-dominant diseases. We discuss the potential therapeutic effects of targeting CSF1R or its ligands on bone diseases, neurological disease, and cancer.
Table 1.
Therapeutic applications of inhibitors and antibodies against CSF1R.
| Name | Form | Targets | Function | Clinical Trial diseases | Reference |
|---|---|---|---|---|---|
| Pexidartinib (PLX3397) | Small molecular inhibitor | CSF1R, c-KIT, VEGFR, and Flt3 | Inhibition of CSF1R signaling | Autoimmune diseases, cancer, and Alzheimer’s disease | 172,193,195,198,204,206 |
| Imatinib | Small molecular inhibitor | CSF1R, ABL, c-KIT, and PDGFR-β | Inhibition of CSF1R kinase activity | Osteoporosis, osteolysis, chronic myeloid leukemia (CML), and breast cancer | 153,168,169 |
| PLX5622 | Small molecular inhibitor | CSF1R | Inhibition of CSF1R signaling | Rheumatoid arthritis, cancer, neuropathic pain, and Alzheimer’s disease | 187–189,191,194,196 |
| BLZ945 | Small molecular inhibitor | CSF1R, c-Kit, PDGFRβ, and Flt3 | Inhibition of CSF1R signaling | Cancer and amyotrophic lateral sclerosis | 197,207,208 |
| GW2580 | Small molecular inhibitor | CSF1R | Inhibition of CSF1R kinase activity | Arthritis, osteoporosis, and cancer | 165,190,203,210–212 |
| Ki20227 | Small molecular inhibitor | CSF1R, VEGFR2, c-KIT, and PDGFRβ | Inhibition of CSF1R kinase activity | Osteolytic bone destruction and breast cancer | 164,183 |
| Edicotinib (JNJ-40346527) | Small molecular inhibitor | CSF1R, KIT, and Flt3 | Inhibition of CSF1R signaling | Alzheimer’s disease, rheumatoid arthritis, and neurodegenerative diseases | 173,174,186 |
| AFS98 (anti-mouse CSF1R) | Monoclonal antibody | CSF1R | Blockade of CSF1R | Cancer, arthritis, and diabetic nephropathy | 167,217,218 |
| M279 (anti-mouse CSF1R) | Monoclonal antibody | CSF1R | Blockade of CSF1R | Cancer, arthritis, and bone loss | 177 |
Bone diseases
In the field of bone research, the suitability of targeting CSF1R in treating bone diseases is continually being investigated. Blockade or depletion of CSF1R suppresses the formation and activity of osteoclasts and attenuates pathological bone resorption in inflammatory arthritis, inflammatory bone destruction, and osteoporosis. Serum IL-34 and CSF1 are linked to the disease activity in patients with rheumatoid arthritis and osteoporosis. Blocking the activation of CSF1R by CSF1R inhibitors such as Ki20227164 and GW2580165 or by antibodies such as anti-CSF1 antibodies166 and AFS98167 attenuates the progression of joint inflammation, bone erosion, and systemic bone erosion in animal models of arthritis. Administration of anti-CSF1R antibodies suppresses osteoclastogenesis and bone resorption in both serum-induced inflammatory arthritis and TNFα-induced inflammatory ostelysis122. Imatinib, a tyrosine kinase inhibitor, prevents and treats arthritis induced by type II collagen antibody (CAIA) and collagen-induced arthritis153,168. Imatinib also suppresses CSF1R expression169 and enhances mature osteoclast apoptosis. Imatinib decreases the proliferation of rheumatoid arthritis synovial cells, suggesting that CSF1R inhibition could potentially mitigate rheumatoid arthritis-induced bone damage170,171. The CSF1R inhibitor PLX3397 significantly inhibits lipopolysaccharide (LPS)-induced bone erosion and the reduction in biomechanical properties in a rat model172. Injection of CSF1R neutralizing antibodies, which were previously shown to inhibit LPS-induced osteoclastogenesis in vivo, reduces orthodontic relapse in mouse models173. However, the administration of JNJ-40346527 to active rheumatoid arthritis patients for 12 weeks does not show any efficacy, despite the increase in the levels of circulating CSF1174. The effect of JNJ-40346527 on bone erosion in the same clinical trial remains unknown. In addition, the effect of small molecules that regulate CSF1R degradation or expression on inflammatory arthritis has also been investigated. Proteasome inhibitors such as MG132 and bortezomib, which can inhibit osteoclast differentiation, also ameliorate LPS-induced bone degradation in mice potentially by accelerating the degradation of CSF1R175. Downregulation of CSF1R and receptor activator of NF-kB (RANK) using extracellular binding immunoglobulin protein (BiP) reduces inflammation and bone loss in the human tumor necrosis factor transgenic (hTNFtg) mouse model176.
CSF1R-mediated signaling contributes to the pathophysiology of osteoporosis, and recent studies show its potential applicability in treating bone diseases such as osteoporosis. Systemic administration of anti-CSF1R antibodies ablates osteoclasts, increases bone density, and prevents mice from age-induced bone loss177. Anti-CSF1R antibodies also deplete tissue macrophages from many different organs and further block the replenishment of macrophages178. Neutralizing CSF1 in vivo completely protects mice from ovariectomy (OVX)-induced bone loss179. Selective deletion of the double isoform of CSF1 also ameliorates OVX-induced bone loss in mice180. Moreover, a newly developed bispecific inhibitor of CSF1R and αvβ3 integrin specifically inhibits osteoclast activity in vitro and reduces serum CTX-1 in ovariectomized mice181. In addition, CSF1R-mediated signaling has been shown to mediate osteolysis in metastatic tumors. Serum CSF1 is increased in patients with lung cancer, and knockdown of CSF1 reduces osteoclasts and improves bone metastasis182. Gorham‐Stout disease (GSD) is a rare bone disease characterized by massive osteolysis associated with elevated levels of CSF1 produced by lymphatic endothelial cells (LECs), and the CSF1R inhibitor Ki20227 suppresses bone destruction in LEC-induced osteolysis183. Although the effect of CSF1R inhibitors on cancer has been extensively explored and is described below, their effect on osteolysis of metastatic tumors has not been well documented.
Neurological diseases
In the brain, CSF1R is abundant in microglia and other glial cells. Microglia have myriad roles in the nervous system and are consequently studied for their potential use in a wide variety of neurological conditions, ranging from Alzheimer’s disease to epilepsy184. Microglia are dependent on CSF1R for survival and proliferation185. CSF1R inhibitors have also been used to examine the roles of microglia during neurological development and healing processes. Intriguingly, anti-IL-34 and anti-CSF1 differentially deplete microglia in the gray and white matter of the brain139, and CSF1R inhibitors such as JNJ-40346527 attenuate microglial proliferation186. Elimination of embryonic microglia with PLX5622 increased weight gain, reduced the number of pro-opiomelanocortin (POMC) neurons, resulted in abnormal cranial and dental formation, and exhibited long-term sex-specific effects, suggesting that microglia could play crucial roles in the early development of the hypothalamus187. Moreover, inhibition of microglia with low doses of PLX5622 or long-term oral treatment did not significantly affect the number of oligodendrocyte progenitor cells (OPCs) ex vivo and in vivo in mouse models, indicating that microglia may not be essential for OPC viability188. Moreover, depletion of microglia during laser-induced choroidal neovascularization (CNV) in PLX5622-treated mice accelerated the ablation of CNV lesion size and decreased macrophage accumulation in the laser site189. Similarly, chronic CSF1R inhibition with GW2580 reduced spinal cord injury-induced microglial/macrophage proliferation in mice and improved the recovery of fine motor control190. Finally, PLX5622 significantly mitigated neuropathic pain in mice induced by partial sciatic nerve ligation by reducing the accumulation of macrophages in the damaged nerves, inhibiting CD86+ M1-like macrophages, and attenuating the expression of proinflammatory cytokines such as TNF-α191.
Increased proinflammatory cytokine levels and increased CSF1 have been found in Parkinson’s disease (PD) patients192. CSF1R inhibitors can deplete microglia. Early long-term PLX3397 treatment of 5XFAD mice, which demonstrates significant neuronal loss similar to that of Alzheimer’s disease, significantly decreases amyloid accumulation and neuritic plaque disposition by decreasing microglial cells193. The newly synthesized brain-penetrating CSF1R inhibitor PLX5622 also decreases parenchymal plaque formation in a 5XFAD mouse model by inducing long-term microglial depletion194. Similarly, treatment with PLX3397 causes microglial depletion and consequently improves sensory motor functions and depressive-like behavior in mice that were administered 6-hydroxydopamine, which induces pathological symptoms of Parkinson’s disease such as the destruction of dopaminergic neurons195. Administration of PLX5622 in an experimental murine autoimmune encephalomyelitis model of multiple sclerosis (MS) attenuates the microglial population and significantly decreases the symptoms of MS, such as demyelination196. Treatment with the CSF1R kinase inhibitor BLZ945 also increased remyelination specifically in the striatum/cortex and decreased demyelination in the corpus callosum in the murine cuprizone demyelination model by ablating microglia and increasing oligodendrocytes197. Moreover, CSF1R inhibition by PLX3397 in epileptic mice significantly reduces the frequency of seizures by affecting module 18 expression and macrophage phenotype without depleting microglial cells198.
Cancer
Aberrant expression of CSF1R contributes to the development of cancer, including Hodgkin lymphoma and anaplastic large cell lymphoma199. Increasing evidence supports that CSF1R affects tumor-associated macrophages (TAMs) to promote tumor progression. TAMs are alternatively activated macrophages that infiltrate into tumors and exhibit anti-inflammatory and protumorigenic phenotypes. TAMs contribute to tumor progression at multiple levels. Blocking CSF1R inhibits the accumulation of immunosuppressive TAMs. Metastasis and TAM infiltration of spontaneous MMTV-PyMT breast tumors are delayed in CSF1R-deficient op/op mice, providing the first evidence for the effect of CSF1R on TAMs200. Moreover, CSF1R-positive macrophages correlate with poor survival of patients with lymphoma and solid tumors201,202. A study using GW2580 shows that inhibiting CSF1R could impair the progression of hepatocellular carcinoma in mice203. While it has been demonstrated that inhibiting CSF1R via PLX3397 (pexidartinib)204 has antitumor effects in adult T-cell leukemia/lymphoma (ATLL) by inducing ATL-T cells to undergo apoptosis and reducing the expression of PD-L1/L2205, another study shows that PLX3397 induces pancreatic ductal adenocarcinoma by upregulating T-cell checkpoint molecules206. BLZ945 inhibits glioma progression207 and delays cervical and mammary tumor growth208. Furthermore, eliminating interstitial macrophages by blocking CSF1R with the anti-CSF1R antibody CS7 prevents radiation pulmonary fibrosis209.
Furthermore, high-impact breakthroughs in the field of CSF1R inhibitors have identified the mechanisms by which CSF1R-expressing cells can promote the growth of cancer cells. In acute myeloid leukemia (AML), cells that express CSF1R support cancer cells by secreting hepatocyte growth factor (HGF) and other cytokines that help cancer cell survival and proliferation. Consequently, GW-2580 decreases the viability of AML patient samples, while resistance to GW-2580 is linked to decreased overall survival210. In mantle cell lymphoma (MCL), primary MCL cells transform monocytes into specific CD163+ M2-like macrophages by secreting CSF1 and IL-10. These macrophages, in turn, increase the survival and proliferation of MCL cells, and GW2580 reduces MCL cell viability in samples that are both resistant and nonresistant to the BTK inhibitor ibrutinib211. In chronic lymphocytic leukemia (CLL), CD14-positive cells that express CSF1R support CCL cell survival, and treatment with GW2580 or ARRY-382 decreases CCL cell viability212. In multiple myeloma (MM), blocking CSF1R with CS7 inhibits the proliferation and differentiation of M2 macrophages and myeloma-associated macrophages (MAMs). In the murine MM model, a CSF1R inhibitor demonstrates a therapeutic effect against cancer by repolarizing MAMs to adopt an M1-like phenotype and inducing a cytotoxic CD4+ T-cell response against tumor cells213.
However, the use of CSF1R inhibitors alone as a cancer treatment has also drawn conflicting conclusions. Single-cell RNA sequencing analysis reveals the presence two different TAM populations in colorectal cancer patients: tumor-suppressive TAMs, which have inflammatory gene signatures, and tumor-promoting polymorphonuclear myeloid-derived suppressor cells (PMN-MDSCs), which have an angiogenic gene signature214. Treatment with anti-CSF1R depletes only TAMs with an inflammatory gene signature but not PMN-MDSCs in colorectal cancer patients, leading to cancer-promoting effects through the accumulation of tumor-infiltrating PMN-MDSCs, which aggregated in tumors. In turn, simultaneous treatment with both a CSF1R inhibitor and a CXCR2 inhibitor targeting PMN-MDSCs effectively decreases tumor growth215. In two breast cancer models, CSF1 neutralization increases spontaneous metastasis without altering primary tumor growth in mice216. Targeting CSF1R by imatinib or AFS98 inhibits bone metastases in breast cancer by suppressing osteoclasts217,218. Another study also shows that simultaneous inhibition of CSF1R and SHP2 via dual-inhibitor-loaded nanoparticles (DNTs) is effective in mitigating tumors in breast cancer and melanoma mouse models219. Furthermore, dual inhibition of CSF1R and MAP kinase signaling also results in reduced tumor growth by reprogramming M2 macrophages to adopt the M1 phenotype220. The beneficial effects of targeting CSF1R, along with other therapeutic interventions, need to be further investigated.
Clinical safety of inhibiting CSF1R
Although CSF1R deficiency in mice leads to significant defects in the functions of macrophages and osteoclasts, targeting CSF1R via small molecule inhibitors and monoclonal antibodies is relatively well tolerated and shows manageable adverse events. The dose-limiting toxicities of CSF1R inhibition have also been reported in only a few studies (reviewed in ref. 221). Several safety studies of CSF1R inhibition in healthy subjects, patients with rheumatoid arthritis, and patients with advanced or metastatic cancers, including giant cell tumors, glioblastoma, and Hodgkin lymphoma, have been reported174,222–225. Although there are some differences between small molecule inhibitors and monoclonal antibodies, the commonly reported adverse events for both inhibitors and antibodies are fatigue, nausea/vomiting, facial and peripheral edema, asthenia, pruritus, rash, headache, dry skin, increased lacrimation, and decreased appetite226. However, serious adverse events such as periorbital edema, lupus erythematosus, erythema, and dermo-hypodermatis have also been reported in trials using monoclonal antibodies to block CSF1R activation227. Nonfatal liver toxicity and elevated liver enzymes such as aspartate aminotransferase (ALT) and alanine transaminase (ALT) have been reported in clinical trials of CSF1R inhibition and may result from the depletion of CSF1R-expressing Kupffer cells from the liver or from targeting other receptors such as KIT. Although increased liver enzymes are short-lived or tolerated in most studies, a phase III study of pexidartinib was suspended due to fatal liver toxicity (NCT02371369). In clinical studies, the effect of CSF1R inhibition on bone health has not been well characterized. A FPA008 trial in 12 rheumatoid arthritis patients and a phase 1 study of ARRY-382 in cancer patients have been reported as meeting abstracts and show that CSF1R inhibition leads to increased serum CSF1 and/or IL-34 levels and diminished NTX228,229. How CSF1R inhibition affects osteoclast precursor cells and osteoclasts remains unknown, and the effect of CSF1R inhibition on bone remodeling needs to be further evaluated in human studies.
Perspectives
Considerable research conducted over the past decades has identified the function of CSF1R-mediated signaling under physiological and pathological conditions. IL-34 and CSF1 are increased in pathological conditions and are considered potential biomarkers for disease prognosis and/or therapeutic targets. Over the past several years, the biological characteristics of IL‐34 and the importance of the IL‐34 signaling network in health and disease have been discovered. Although the binding of CSF1R with IL-34 and CSF1 activates similar signaling pathways, IL-34 and CSF1 have overlapping but distinct binding to CSF1R. IL-34 and CSF1 show functional differences and activate downstream signals with different intensities and differential targets. Therefore, understanding the differential mechanisms of CSF1- or IL-34-mediated regulation of osteoclastogenesis will be important, and CSF1R-targeting strategies in bone diseases may need to take into account the effects of alterations in IL-34-mediated signaling pathways.
Targeting CSF1R has gained much attention in many disease conditions in which myeloid cells play an important role. There have been several clinical trials targeting CSF1R or CSF1. However, recent clinical trials targeting CSF1R in inflammatory diseases or cancer have not been promising161. Emerging data address the potential mechanism by which CSF1R blockade has been ineffective in inflammation and cancer. CSF1R-expressing cells may play an anti-inflammatory role or a cancer-suppressive role. CSF1 might negatively regulate inflammatory responses by activating PI3K230, and CSF1R is expressed on both tumor-suppressing and tumor-promoting myeloid cells215. Thus, it is still necessary to clarify whether targeting CSF1R will be beneficial for clinical intervention in osteoclast-mediated pathological bone destruction, such as inflammatory bone destruction and bone osteolysis in tumors. Given that CSF1R plays a critical role in most myeloid lineage cells, a better understanding of how CSF1R regulates disease progression is critical for developing a specific therapeutic interventions or appropriate therapeutic strategies.
Acknowledgements
This work was supported by the National Institutes of Health (5R01 AR069562 and 5R01 AR073156 to P.M.K.H.). Figures were generated by www.biorender.com.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tsukasaki M, Takayanagi H. Osteoimmunology: evolving concepts in bone-immune interactions in health and disease. Nat. Rev. Immunol. 2019;19:626–642. doi: 10.1038/s41577-019-0178-8. [DOI] [PubMed] [Google Scholar]
- 2.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 3.Feng X, Teitelbaum SL. Osteoclasts: New Insights. Bone Res. 2013;1:11–26. doi: 10.4248/BR201301003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park-Min KH. Mechanisms involved in normal and pathological osteoclastogenesis. Cell Mol. Life Sci. 2018;75:2519–2528. doi: 10.1007/s00018-018-2817-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Novack DV, Teitelbaum SL. The osteoclast: friend or foe? Annu. Rev. Pathol. 2008;3:457–484. doi: 10.1146/annurev.pathmechdis.3.121806.151431. [DOI] [PubMed] [Google Scholar]
- 6.Tagoh H, et al. Transcription factor complex formation and chromatin fine structure alterations at the murine c-fms (CSF-1 receptor) locus during maturation of myeloid precursor cells. Genes Dev. 2002;16:1721–1737. doi: 10.1101/gad.222002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamilton JA. Colony-stimulating factors in inflammation and autoimmunity. Nat. Rev. Immunol. 2008;8:533–544. doi: 10.1038/nri2356. [DOI] [PubMed] [Google Scholar]
- 8.Ross FP, Teitelbaum SL. alphavbeta3 and macrophage colony-stimulating factor: partners in osteoclast biology. Immunol. Rev. 2005;208:88–105. doi: 10.1111/j.0105-2896.2005.00331.x. [DOI] [PubMed] [Google Scholar]
- 9.Pixley FJ, Stanley ER. CSF-1 regulation of the wandering macrophage: complexity in action. Trends Cell Biol. 2004;14:628–638. doi: 10.1016/j.tcb.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 10.Fixe P, Praloran V. M-CSF: haematopoietic growth factor or inflammatory cytokine? Cytokine. 1998;10:32–37. doi: 10.1006/cyto.1997.0249. [DOI] [PubMed] [Google Scholar]
- 11.Ross FP. M-CSF, c-Fms, and signaling in osteoclasts and their precursors. Ann. N. Y. Acad. Sci. 2006;1068:110–116. doi: 10.1196/annals.1346.014. [DOI] [PubMed] [Google Scholar]
- 12.Arai F, et al. Commitment and differentiation of osteoclast precursor cells by the sequential expression of c-fms and receptor activator of nuclear factor κB (RANK) receptors. J. Exp. Med. 1999;190:1741–1754. doi: 10.1084/jem.190.12.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat. Rev. Immunol. 2007;7:292–304. doi: 10.1038/nri2062. [DOI] [PubMed] [Google Scholar]
- 14.Park-Min KH. Metabolic reprogramming in osteoclasts. Semin Immunopathol. 2019;41:565–572. doi: 10.1007/s00281-019-00757-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yasuda H, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc. Natl Acad. Sci. USA. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takayanagi H, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Developmental Cell. 2002;3:889–901. doi: 10.1016/s1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- 17.Simonet WS, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 18.Humphrey MB, Nakamura MC. A comprehensive review of immunoreceptor regulation of osteoclasts. Clin. Rev. Allergy Immunol. 2016;51:48–58. doi: 10.1007/s12016-015-8521-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Humphrey MB, et al. The signaling adapter protein DAP12 regulates multinucleation during osteoclast development. J. Bone Min. Res. 2004;19:224–234. doi: 10.1359/JBMR.0301234. [DOI] [PubMed] [Google Scholar]
- 20.Koga T, et al. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature. 2004;428:758–763. doi: 10.1038/nature02444. [DOI] [PubMed] [Google Scholar]
- 21.Faccio R, Zou W, Colaianni G, Teitelbaum SL, Ross FP. High dose M-CSF partially rescues the Dap12-/- osteoclast phenotype. J. Cell Biochem. 2003;90:871–883. doi: 10.1002/jcb.10694. [DOI] [PubMed] [Google Scholar]
- 22.Mocsai A, et al. The immunomodulatory adapter proteins DAP12 and Fc receptor gamma-chain (FcRgamma) regulate development of functional osteoclasts through the Syk tyrosine kinase. Proc. Natl Acad. Sci. USA. 2004;101:6158–6163. doi: 10.1073/pnas.0401602101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Otero K, et al. Macrophage colony-stimulating factor induces the proliferation and survival of macrophages via a pathway involving DAP12 and beta-catenin. Nat. Immunol. 2009;10:734–743. doi: 10.1038/ni.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zou W, Reeve JL, Liu Y, Teitelbaum SL, Ross FP. DAP12 couples c-Fms activation to the osteoclast cytoskeleton by recruitment of Syk. Mol. Cell. 2008;31:422–431. doi: 10.1016/j.molcel.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chitu V, Stanley ER. Colony-stimulating factor-1 in immunity and inflammation. Curr. Opin. Immunol. 2006;18:39–48. doi: 10.1016/j.coi.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 26.Pollard JW. Trophic macrophages in development and disease. Nat. Rev. Immunol. 2009;9:259–270. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshida H, et al. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 1990;345:442–444. doi: 10.1038/345442a0. [DOI] [PubMed] [Google Scholar]
- 28.Kodama H, et al. Congenital osteoclast deficiency in osteopetrotic (op/op) mice is cured by injections of macrophage colony-stimulating factor. J. Exp. Med. 1991;173:269–272. doi: 10.1084/jem.173.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dai XM, et al. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood. 2002;99:111–120. doi: 10.1182/blood.v99.1.111. [DOI] [PubMed] [Google Scholar]
- 30.Begg SK, et al. Delayed hematopoietic development in osteopetrotic (op/op) mice. J. Exp. Med. 1993;177:237–242. doi: 10.1084/jem.177.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin H, et al. Discovery of a cytokine and its receptor by functional screening of the extracellular proteome. Science. 2008;320:807–811. doi: 10.1126/science.1154370. [DOI] [PubMed] [Google Scholar]
- 32.Chen Z, Buki K, Vaaraniemi J, Gu G, Vaananen HK. The critical role of IL-34 in osteoclastogenesis. PLoS ONE. 2011;6:e18689. doi: 10.1371/journal.pone.0018689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoeffel G, et al. C-Myb(+) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity. 2015;42:665–678. doi: 10.1016/j.immuni.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lichanska AM, et al. Differentiation of the mononuclear phagocyte system during mouse embryogenesis: the role of transcription factor PU.1. Blood. 1999;94:127–138. [PubMed] [Google Scholar]
- 35.Yahara Y, et al. Erythromyeloid progenitors give rise to a population of osteoclasts that contribute to bone homeostasis and repair. Nat. Cell Biol. 2020;22:49–59. doi: 10.1038/s41556-019-0437-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacome-Galarza CE, et al. Developmental origin, functional maintenance and genetic rescue of osteoclasts. Nature. 2019;568:541–545. doi: 10.1038/s41586-019-1105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Novak S, et al. Osteoclasts derive predominantly from bone marrow-resident CX3CR1(+) precursor cells in homeostasis, whereas circulating CX3CR1(+) cells contribute to osteoclast development during fracture repair. J. Immunol. 2020;204:868–878. doi: 10.4049/jimmunol.1900665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rademakers R, et al. Mutations in the colony stimulating factor 1 receptor (CSF1R) gene cause hereditary diffuse leukoencephalopathy with spheroids. Nat. Genet. 2011;44:200–205. doi: 10.1038/ng.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nicholson AM, et al. CSF1R mutations link POLD and HDLS as a single disease entity. Neurology. 2013;80:1033–1040. doi: 10.1212/WNL.0b013e31828726a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Foulds N, et al. Adult-onset leukoencephalopathy with axonal spheroids and pigmented glia caused by a novel R782G mutation in CSF1R. Sci. Rep. 2015;5:10042. doi: 10.1038/srep10042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oosterhof N, et al. Homozygous mutations in CSF1R cause a pediatric-onset leukoencephalopathy and can result in congenital absence of microglia. Am. J. Hum. Genet. 2019;104:936–947. doi: 10.1016/j.ajhg.2019.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chitu V, et al. Phenotypic characterization of a Csf1r haploinsufficient mouse model of adult-onset leukodystrophy with axonal spheroids and pigmented glia (ALSP) Neurobiol. Dis. 2015;74:219–228. doi: 10.1016/j.nbd.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Konno T, Kasanuki K, Ikeuchi T, Dickson DW, Wszolek ZK. CSF1R-related leukoencephalopathy: a major player in primary microgliopathies. Neurology. 2018;91:1092–1104. doi: 10.1212/WNL.0000000000006642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hofer TP, et al. slan-defined subsets of CD16-positive monocytes: impact of granulomatous inflammation and M-CSF receptor mutation. Blood. 2015;126:2601–2610. doi: 10.1182/blood-2015-06-651331. [DOI] [PubMed] [Google Scholar]
- 45.Askew K, et al. Coupled proliferation and apoptosis maintain the rapid turnover of microglia in the adult brain. Cell Rep. 2017;18:391–405. doi: 10.1016/j.celrep.2016.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guo L, et al. Bi-allelic CSF1R mutations cause skeletal dysplasia of dysosteosclerosis-pyle disease spectrum and degenerative encephalopathy with brain malformation. Am. J. Hum. Genet. 2019;104:925–935. doi: 10.1016/j.ajhg.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Le Beau MM, et al. Assignment of the GM-CSF, CSF-1, and FMS genes to human chromosome 5 provides evidence for linkage of a family of genes regulating hematopoiesis and for their involvement in the deletion (5q) in myeloid disorders. Cold Spring Harb. Symp. Quant. Biol. 1986;51:899–909. doi: 10.1101/sqb.1986.051.01.103. [DOI] [PubMed] [Google Scholar]
- 48.Hoggan MD, Halden NF, Buckler CE, Kozak CA. Genetic mapping of the mouse c-fms proto-oncogene to chromosome 18. J. Virol. 1988;62:1055–1056. doi: 10.1128/jvi.62.3.1055-1056.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ovchinnikov DA, DeBats CE, Sester DP, Sweet MJ, Hume DA. A conserved distal segment of the mouse CSF-1 receptor promoter is required for maximal expression of a reporter gene in macrophages and osteoclasts of transgenic mice. J. Leukoc. Biol. 2010;87:815–822. doi: 10.1189/jlb.0809557. [DOI] [PubMed] [Google Scholar]
- 50.Rojo R, Pridans C, Langlais D, Hume DA. Transcriptional mechanisms that control expression of the macrophage colony-stimulating factor receptor locus. Clin. Sci. (Lond.) 2017;131:2161–2182. doi: 10.1042/CS20170238. [DOI] [PubMed] [Google Scholar]
- 51.Bonifer C, Hume DA. The transcriptional regulation of the colony-stimulating factor 1 receptor (csf1r) gene during hematopoiesis. Front Biosci. 2008;13:549–560. doi: 10.2741/2700. [DOI] [PubMed] [Google Scholar]
- 52.Yue X, Favot P, Dunn TL, Cassady AI, Hume DA. Expression of mRNA encoding the macrophage colony-stimulating factor receptor (c-fms) is controlled by a constitutive promoter and tissue-specific transcription elongation. Mol. Cell Biol. 1993;13:3191–3201. doi: 10.1128/mcb.13.6.3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Himes SR, et al. A highly conserved c-fms gene intronic element controls macrophage-specific and regulated expression. J. Leukoc. Biol. 2001;70:812–820. [PubMed] [Google Scholar]
- 54.Sauter KA, et al. The function of the conserved regulatory element within the second intron of the mammalian Csf1r locus. PLoS ONE. 2013;8:e54935. doi: 10.1371/journal.pone.0054935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Follows GA, et al. c-FMS chromatin structure and expression in normal and leukaemic myelopoiesis. Oncogene. 2005;24:3643–3651. doi: 10.1038/sj.onc.1208655. [DOI] [PubMed] [Google Scholar]
- 56.Rojo R, et al. Deletion of a Csf1r enhancer selectively impacts CSF1R expression and development of tissue macrophage populations. Nat. Commun. 2019;10:3215. doi: 10.1038/s41467-019-11053-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu H, et al. The mechanism of shared but distinct CSF-1R signaling by the non-homologous cytokines IL-34 and CSF-1. Biochim Biophys. Acta. 2012;1824:938–945. doi: 10.1016/j.bbapap.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stanley, E. R. & Chitu, V. CSF-1 receptor signaling in myeloid cells. Cold Spring Harb Perspect Biol6, 10.1101/cshperspect.a021857 (2014). [DOI] [PMC free article] [PubMed]
- 59.Da Silva Figueiredo Celestino Gomes, P. et al. Differential effects of CSF-1R D802V and KIT D816V homologous mutations on receptor tertiary structure and allosteric communication. PLoS ONE. 2014;9:e97519. doi: 10.1371/journal.pone.0097519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sasmono RT, et al. A macrophage colony-stimulating factor receptor-green fluorescent protein transgene is expressed throughout the mononuclear phagocyte system of the mouse. Blood. 2003;101:1155–1163. doi: 10.1182/blood-2002-02-0569. [DOI] [PubMed] [Google Scholar]
- 61.Sasmono RT, Williams E. Generation and characterization of MacGreen mice, the Cfs1r-EGFP transgenic mice. Methods Mol. Biol. 2012;844:157–176. doi: 10.1007/978-1-61779-527-5_11. [DOI] [PubMed] [Google Scholar]
- 62.Hawley CA, et al. Csf1r-mApple transgene expression and ligand binding in vivo reveal dynamics of CSF1R expression within the mononuclear phagocyte system. J. Immunol. 2018;200:2209–2223. doi: 10.4049/jimmunol.1701488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nandi S, et al. The CSF-1 receptor ligands IL-34 and CSF-1 exhibit distinct developmental brain expression patterns and regulate neural progenitor cell maintenance and maturation. Dev. Biol. 2012;367:100–113. doi: 10.1016/j.ydbio.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arceci RJ, Pampfer S, Pollard JW. Expression of CSF-1/c-fms and SF/c-kit mRNA during preimplantation mouse development. Dev. Biol. 1992;151:1–8. doi: 10.1016/0012-1606(92)90207-w. [DOI] [PubMed] [Google Scholar]
- 65.Mancini A, et al. Identification of a second Grb2 binding site in the v-Fms tyrosine kinase. Oncogene. 1997;15:1565–1572. doi: 10.1038/sj.onc.1201518. [DOI] [PubMed] [Google Scholar]
- 66.Yu W, et al. Macrophage proliferation is regulated through CSF-1 receptor tyrosines 544, 559, and 807. J. Biol. Chem. 2012;287:13694–13704. doi: 10.1074/jbc.M112.355610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Levkowitz G, et al. Ubiquitin ligase activity and tyrosine phosphorylation underlie suppression of growth factor signaling by c-Cbl/Sli-1. Mol. Cell. 1999;4:1029–1040. doi: 10.1016/s1097-2765(00)80231-2. [DOI] [PubMed] [Google Scholar]
- 68.Rohde CM, Schrum J, Lee AW. A juxtamembrane tyrosine in the colony stimulating factor-1 receptor regulates ligand-induced Src association, receptor kinase function, and down-regulation. J. Biol. Chem. 2004;279:43448–43461. doi: 10.1074/jbc.M314170200. [DOI] [PubMed] [Google Scholar]
- 69.Xiong Y, et al. A CSF-1 receptor phosphotyrosine 559 signaling pathway regulates receptor ubiquitination and tyrosine phosphorylation. J. Biol. Chem. 2011;286:952–960. doi: 10.1074/jbc.M110.166702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Takeshita S, et al. c-Fms tyrosine 559 is a major mediator of M-CSF-induced proliferation of primary macrophages. J. Biol. Chem. 2007;282:18980–18990. doi: 10.1074/jbc.M610938200. [DOI] [PubMed] [Google Scholar]
- 71.van der Geer P, Hunter T. Mutation of Tyr697, a GRB2-binding site, and Tyr721, a PI 3-kinase binding site, abrogates signal transduction by the murine CSF-1 receptor expressed in Rat-2 fibroblasts. EMBO J. 1993;12:5161–5172. doi: 10.1002/j.1460-2075.1993.tb06211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pawson T, Schlessingert J. SH2 and SH3 domains. Curr. Biol. 1993;3:434–442. doi: 10.1016/0960-9822(93)90350-w. [DOI] [PubMed] [Google Scholar]
- 73.Bourette RP, et al. Mona, a novel hematopoietic-specific adaptor interacting with the macrophage colony-stimulating factor receptor, is implicated in monocyte/macrophage development. EMBO J. 1998;17:7273–7281. doi: 10.1093/emboj/17.24.7273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang Y, Yeung YG, Stanley ER. CSF-1 stimulated multiubiquitination of the CSF-1 receptor and of Cbl follows their tyrosine phosphorylation and association with other signaling proteins. J. Cell Biochem. 1999;72:119–134. [PubMed] [Google Scholar]
- 75.Reedijk M, et al. Tyr721 regulates specific binding of the CSF-1 receptor kinase insert to PI 3’-kinase SH2 domains: a model for SH2-mediated receptor-target interactions. EMBO J. 1992;11:1365–1372. doi: 10.1002/j.1460-2075.1992.tb05181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Novak U, Nice E, Hamilton JA, Paradiso L. Requirement for Y706 of the murine (or Y708 of the human) CSF-1 receptor for STAT1 activation in response to CSF-1. Oncogene. 1996;13:2607–2613. [PubMed] [Google Scholar]
- 77.Yu W, et al. CSF-1 receptor structure/function in MacCsf1r-/- macrophages: regulation of proliferation, differentiation, and morphology. J. Leukoc. Biol. 2008;84:852–863. doi: 10.1189/jlb.0308171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bourette RP, Myles GM, Choi JL, Rohrschneider LR. Sequential activation of phoshatidylinositol 3-kinase and phospholipase C-gamma2 by the M-CSF receptor is necessary for differentiation signaling. EMBO J. 1997;16:5880–5893. doi: 10.1093/emboj/16.19.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hanks SK, Quinn AM. Protein kinase catalytic domain sequence database: identification of conserved features of primary structure and classification of family members. Methods Enzymol. 1991;200:38–62. doi: 10.1016/0076-6879(91)00126-h. [DOI] [PubMed] [Google Scholar]
- 80.Joos H, Trouliaris S, Helftenbein G, Niemann H, Tamura T. Tyrosine phosphorylation of the juxtamembrane domain of the v-Fms oncogene product is required for its association with a 55-kDa protein. J. Biol. Chem. 1996;271:24476–24481. doi: 10.1074/jbc.271.40.24476. [DOI] [PubMed] [Google Scholar]
- 81.Lee PS, et al. The Cbl protooncoprotein stimulates CSF-1 receptor multiubiquitination and endocytosis, and attenuates macrophage proliferation. EMBO J. 1999;18:3616–3628. doi: 10.1093/emboj/18.13.3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tushinski RJ, et al. Survival of mononuclear phagocytes depends on a lineage-specific growth factor that the differentiated cells selectively destroy. Cell. 1982;28:71–81. doi: 10.1016/0092-8674(82)90376-2. [DOI] [PubMed] [Google Scholar]
- 83.Huynh J, Kwa MQ, Cook AD, Hamilton JA, Scholz GM. CSF-1 receptor signalling from endosomes mediates the sustained activation of Erk1/2 and Akt in macrophages. Cell Signal. 2012;24:1753–1761. doi: 10.1016/j.cellsig.2012.04.022. [DOI] [PubMed] [Google Scholar]
- 84.Tushinski RJ, Stanley ER. The regulation of macrophage protein turnover by a colony stimulating factor (CSF-1) J. Cell Physiol. 1983;116:67–75. doi: 10.1002/jcp.1041160111. [DOI] [PubMed] [Google Scholar]
- 85.Pixley FJ. Macrophage migration and its regulation by CSF-1. Int. J. Cell Biol. 2012;2012:501962. doi: 10.1155/2012/501962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pixley FJ, Lee PS, Condeelis JS, Stanley ER. Protein tyrosine phosphatase phi regulates paxillin tyrosine phosphorylation and mediates colony-stimulating factor 1-induced morphological changes in macrophages. Mol. Cell Biol. 2001;21:1795–1809. doi: 10.1128/MCB.21.5.1795-1809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dai XM, Zong XH, Akhter MP, Stanley ER. Osteoclast deficiency results in disorganized matrix, reduced mineralization, and abnormal osteoblast behavior in developing bone. J. Bone Min. Res. 2004;19:1441–1451. doi: 10.1359/JBMR.040514. [DOI] [PubMed] [Google Scholar]
- 88.Feng X, et al. Tyrosines 559 and 807 in the cytoplasmic tail of the macrophage colony-stimulating factor receptor play distinct roles in osteoclast differentiation and function. Endocrinology. 2002;143:4868–4874. doi: 10.1210/en.2002-220467. [DOI] [PubMed] [Google Scholar]
- 89.Adapala NS, Barbe MF, Langdon WY, Tsygankov AY, Sanjay A. Cbl-phosphatidylinositol 3 kinase interaction differentially regulates macrophage colony-stimulating factor-mediated osteoclast survival and cytoskeletal reorganization. Ann. N. Y. Acad. Sci. 2010;1192:376–384. doi: 10.1111/j.1749-6632.2009.05346.x. [DOI] [PubMed] [Google Scholar]
- 90.Insogna KL, et al. Colony-stimulating factor-1 induces cytoskeletal reorganization and c-src-dependent tyrosine phosphorylation of selected cellular proteins in rodent osteoclasts. J. Clin. Invest. 1997;100:2476–2485. doi: 10.1172/JCI119790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nakamura I, Duong LT, Rodan SB, Rodan GA. Involvement of alpha(v)beta3 integrins in osteoclast function. J. Bone Min. Metab. 2007;25:337–344. doi: 10.1007/s00774-007-0773-9. [DOI] [PubMed] [Google Scholar]
- 92.McHugh KP, et al. Mice lacking beta3 integrins are osteosclerotic because of dysfunctional osteoclasts. J. Clin. Invest. 2000;105:433–440. doi: 10.1172/JCI8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Feng X, et al. A Glanzmann’s mutation in beta 3 integrin specifically impairs osteoclast function. J. Clin. Invest. 2001;107:1137–1144. doi: 10.1172/JCI12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Faccio R, Takeshita S, Zallone A, Ross FP, Teitelbaum SL. c-Fms and the alphavbeta3 integrin collaborate during osteoclast differentiation. J. Clin. Invest. 2003;111:749–758. doi: 10.1172/JCI16924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ji JD, et al. Inhibition of RANK expression and osteoclastogenesis by TLRs and IFN-gamma in human osteoclast precursors. J. Immunol. 2009;183:7223–7233. doi: 10.4049/jimmunol.0900072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee B, et al. Direct inhibition of human RANK+ osteoclast precursors identifies a homeostatic function of IL-1beta. J. Immunol. 2010;185:5926–5934. doi: 10.4049/jimmunol.1001591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ma X, et al. Structural basis for the dual recognition of helical cytokines IL-34 and CSF-1 by CSF-1R. Structure. 2012;20:676–687. doi: 10.1016/j.str.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 98.Nandi S, et al. Receptor-type protein-tyrosine phosphatase zeta is a functional receptor for interleukin-34. J. Biol. Chem. 2013;288:21972–21986. doi: 10.1074/jbc.M112.442731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Segaliny AI, et al. Syndecan-1 regulates the biological activities of interleukin-34. Biochim Biophys. Acta. 2015;1853:1010–1021. doi: 10.1016/j.bbamcr.2015.01.023. [DOI] [PubMed] [Google Scholar]
- 100.Boulakirba S, et al. IL-34 and CSF-1 display an equivalent macrophage differentiation ability but a different polarization potential. Sci. Rep. 2018;8:256. doi: 10.1038/s41598-017-18433-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Paquin-Proulx D, et al. Human interleukin-34-derived macrophages have increased resistance to HIV-1 infection. Cytokine. 2018;111:272–277. doi: 10.1016/j.cyto.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 102.Zhao Z, et al. IL-34 inhibits acute rejection of rat liver transplantation by inducing kupffer cell M2 polarization. Transplantation. 2018;102:e265–e274. doi: 10.1097/TP.0000000000002194. [DOI] [PubMed] [Google Scholar]
- 103.Stanley ER, et al. Biology and action of colony-stimulating factor-1. Mol. Reprod. Dev. 1997;46:4–10. doi: 10.1002/(SICI)1098-2795(199701)46:1<4::AID-MRD2>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 104.Chockalingam S, Ghosh SS. Macrophage colony-stimulating factor and cancer: a review. Tumour Biol. 2014;35:10635–10644. doi: 10.1007/s13277-014-2627-0. [DOI] [PubMed] [Google Scholar]
- 105.Douglass TG, et al. Macrophage colony stimulating factor: not just for macrophages anymore! A gateway into complex biologies. Int. Immunopharmacol. 2008;8:1354–1376. doi: 10.1016/j.intimp.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 106.Cecchini MG, et al. Role of colony stimulating factor-1 in the establishment and regulation of tissue macrophages during postnatal development of the mouse. Development. 1994;120:1357–1372. doi: 10.1242/dev.120.6.1357. [DOI] [PubMed] [Google Scholar]
- 107.Yagiz K, Rittling SR. Both cell-surface and secreted CSF-1 expressed by tumor cells metastatic to bone can contribute to osteoclast activation. Exp. Cell Res. 2009;315:2442–2452. doi: 10.1016/j.yexcr.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Clinton SK, et al. Macrophage colony-stimulating factor gene expression in vascular cells and in experimental and human atherosclerosis. Am. J. Pathol. 1992;140:301–316. [PMC free article] [PubMed] [Google Scholar]
- 109.Elford PR, Felix R, Cecchini M, Trechsel U, Fleisch H. Murine osteoblastlike cells and the osteogenic cell MC3T3-E1 release a macrophage colony-stimulating activity in culture. Calcif. Tissue Int. 1987;41:151–156. doi: 10.1007/BF02563795. [DOI] [PubMed] [Google Scholar]
- 110.Han Y, You X, Xing W, Zhang Z, Zou W. Paracrine and endocrine actions of bone-the functions of secretory proteins from osteoblasts, osteocytes, and osteoclasts. Bone Res. 2018;6:16. doi: 10.1038/s41413-018-0019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stanley ER, Heard PM. Factors regulating macrophage production and growth. Purification and some properties of the colony stimulating factor from medium conditioned by mouse L cells. J. Biol. Chem. 1977;252:4305–4312. [PubMed] [Google Scholar]
- 112.Price LK, Choi HU, Rosenberg L, Stanley ER. The predominant form of secreted colony stimulating factor-1 is a proteoglycan. J. Biol. Chem. 1992;267:2190–2199. [PubMed] [Google Scholar]
- 113.Jang MH, et al. Distinct in vivo roles of colony-stimulating factor-1 isoforms in renal inflammation. J. Immunol. 2006;177:4055–4063. doi: 10.4049/jimmunol.177.6.4055. [DOI] [PubMed] [Google Scholar]
- 114.Wiktor-Jedrzejczak W, et al. Correction by CSF-1 of defects in the osteopetrotic op/op mouse suggests local, developmental, and humoral requirements for this growth factor. Exp. Hematol. 1991;19:1049–1054. [PubMed] [Google Scholar]
- 115.Yao GQ, et al. The cell-surface form of colony-stimulating factor-1 is regulated by osteotropic agents and supports formation of multinucleated osteoclast-like cells. J. Biol. Chem. 1998;273:4119–4128. doi: 10.1074/jbc.273.7.4119. [DOI] [PubMed] [Google Scholar]
- 116.Itoh K, et al. Importance of membrane- or matrix-associated forms of M-CSF and RANKL/ODF in osteoclastogenesis supported by SaOS-4/3 cells expressing recombinant PTH/PTHrP receptors. J. Bone Min. Res. 2000;15:1766–1775. doi: 10.1359/jbmr.2000.15.9.1766. [DOI] [PubMed] [Google Scholar]
- 117.Yao GQ, Sun BH, Weir EC, Insogna KL. A role for cell-surface CSF-1 in osteoblast-mediated osteoclastogenesis. Calcif. Tissue Int. 2002;70:339–346. doi: 10.1007/s00223-001-1079-x. [DOI] [PubMed] [Google Scholar]
- 118.Yao GQ, et al. The cell surface form of colony-stimulating factor-1 is biologically active in bone in vivo. Endocrinology. 2003;144:3677–3682. doi: 10.1210/en.2002-221071. [DOI] [PubMed] [Google Scholar]
- 119.Dai XM, Zong XH, Sylvestre V, Stanley ER. Incomplete restoration of colony-stimulating factor 1 (CSF-1) function in CSF-1-deficient Csf1op/Csf1op mice by transgenic expression of cell surface CSF-1. Blood. 2004;103:1114–1123. doi: 10.1182/blood-2003-08-2739. [DOI] [PubMed] [Google Scholar]
- 120.Bischof RJ, Zafiropoulos D, Hamilton JA, Campbell IK. Exacerbation of acute inflammatory arthritis by the colony-stimulating factors CSF-1 and granulocyte macrophage (GM)-CSF: evidence of macrophage infiltration and local proliferation. Clin. Exp. Immunol. 2000;119:361–367. doi: 10.1046/j.1365-2249.2000.01125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Campbell IK, Rich MJ, Bischof RJ, Hamilton JA. The colony-stimulating factors and collagen-induced arthritis: exacerbation of disease by M-CSF and G-CSF and requirement for endogenous M-CSF. J. Leukoc. Biol. 2000;68:144–150. [PubMed] [Google Scholar]
- 122.Kitaura H, et al. M-CSF mediates TNF-induced inflammatory osteolysis. J. Clin. Invest. 2005;115:3418–3427. doi: 10.1172/JCI26132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kimble RB, Srivastava S, Ross FP, Matayoshi A, Pacifici R. Estrogen deficiency increases the ability of stromal cells to support murine osteoclastogenesis via an interleukin-1and tumor necrosis factor-mediated stimulation of macrophage colony-stimulating factor production. J. Biol. Chem. 1996;271:28890–28897. doi: 10.1074/jbc.271.46.28890. [DOI] [PubMed] [Google Scholar]
- 124.Takei I, et al. High macrophage-colony stimulating factor levels in synovial fluid of loose artificial hip joints. J. Rheumatol. 2000;27:894–899. [PubMed] [Google Scholar]
- 125.Danda R, et al. Proteomic profiling of retinoblastoma by high resolution mass spectrometry. Clin. Proteom. 2016;13:29. doi: 10.1186/s12014-016-9128-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yang PT, et al. Increased expression of macrophage colony-stimulating factor in ankylosing spondylitis and rheumatoid arthritis. Ann. Rheum. Dis. 2006;65:1671–1672. doi: 10.1136/ard.2006.054874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hamilton JA, Tak PP. The dynamics of macrophage lineage populations in inflammatory and autoimmune diseases. Arthritis Rheum. 2009;60:1210–1221. doi: 10.1002/art.24505. [DOI] [PubMed] [Google Scholar]
- 128.Saleh R, et al. CSF-1 in inflammatory and arthritic pain development. J. Immunol. 2018;201:2042–2053. doi: 10.4049/jimmunol.1800665. [DOI] [PubMed] [Google Scholar]
- 129.Alvarado-Vazquez PA, et al. Intra-articular administration of an antibody against CSF-1 receptor reduces pain-related behaviors and inflammation in CFA-induced knee arthritis. Neurosci. Lett. 2015;584:39–44. doi: 10.1016/j.neulet.2014.09.053. [DOI] [PubMed] [Google Scholar]
- 130.Srivastava S, et al. Estrogen blocks M-CSF gene expression and osteoclast formation by regulating phosphorylation of Egr-1 and its interaction with Sp-1. J. Clin. Invest. 1998;102:1850–1859. doi: 10.1172/JCI4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sarma U, Edwards M, Motoyoshi K, Flanagan AM. Inhibition of bone resorption by 17beta-estradiol in human bone marrow cultures. J. Cell Physiol. 1998;175:99–108. doi: 10.1002/(SICI)1097-4652(199804)175:1<99::AID-JCP11>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 132.Scholl SM, et al. Circulating levels of the macrophage colony stimulating factor CSF-1 in primary and metastatic breast cancer patients. A pilot study. Breast Cancer Res. Treat. 1996;39:275–283. doi: 10.1007/BF01806155. [DOI] [PubMed] [Google Scholar]
- 133.McDermott RS, et al. Circulating macrophage colony stimulating factor as a marker of tumour progression. Eur. Cytokine Netw. 2002;13:121–127. [PubMed] [Google Scholar]
- 134.Hsu WC, et al. CSF-1 overexpression predicts poor prognosis in upper tract urothelial carcinomas. Dis. Markers. 2019;2019:2724948. doi: 10.1155/2019/2724948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wei S, et al. Functional overlap but differential expression of CSF-1 and IL-34 in their CSF-1 receptor-mediated regulation of myeloid cells. J. Leukoc. Biol. 2010;88:495–505. doi: 10.1189/jlb.1209822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang Y, et al. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat. Immunol. 2012;13:753–760. doi: 10.1038/ni.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Baghdadi M, et al. Interleukin-34, a comprehensive review. J. Leukoc. Biol. 2018;104:931–951. doi: 10.1002/JLB.MR1117-457R. [DOI] [PubMed] [Google Scholar]
- 138.Lin W, et al. Function of CSF1 and IL34 in macrophage homeostasis, inflammation, and cancer. Front Immunol. 2019;10:2019. doi: 10.3389/fimmu.2019.02019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Easley-Neal C, Foreman O, Sharma N, Zarrin AA, Weimer RM. CSF1R ligands IL-34 and CSF1 are differentially required for microglia development and maintenance in white and gray matter brain regions. Front Immunol. 2019;10:2199. doi: 10.3389/fimmu.2019.02199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lindau R, et al. Interleukin-34 is present at the fetal-maternal interface and induces immunoregulatory macrophages of a decidual phenotype in vitro. Hum. Reprod. 2018;33:588–599. doi: 10.1093/humrep/dey037. [DOI] [PubMed] [Google Scholar]
- 141.Kuil, L. E. et al. Reverse genetic screen reveals that Il34 facilitates yolk sac macrophage distribution and seeding of the brain. Dis. Model Mech.12, 10.1242/dmm.037762 (2019). [DOI] [PMC free article] [PubMed]
- 142.Wu S, et al. Il34-Csf1r pathway regulates the migration and colonization of microglial precursors. Dev. Cell. 2018;46:552–563.e554. doi: 10.1016/j.devcel.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 143.Ogawa S, et al. Interleukin 34 (IL-34) cell-surface localization regulated by the molecular chaperone 78-kDa glucose-regulated protein facilitates the differentiation of monocytic cells. J. Biol. Chem. 2019;294:2386–2396. doi: 10.1074/jbc.RA118.006226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Shen HY, Zhou Y, Zhou QJ, Li MY, Chen J. Mudskipper interleukin-34 modulates the functions of monocytes/macrophages via the colony-stimulating factor-1 receptor 1. Zool. Res. 2020;41:123–137. doi: 10.24272/j.issn.2095-8137.2020.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jiang Y, Chen J, Yen K, Xu J. Ectopically expressed IL-34 can efficiently induce macrophage migration to the liver in zebrafish. Zebrafish. 2019;16:165–170. doi: 10.1089/zeb.2018.1685. [DOI] [PubMed] [Google Scholar]
- 146.Yaparla A, Docter-Loeb H, Melnyk MLS, Batheja A, Grayfer L. The amphibian (Xenopus laevis) colony-stimulating factor-1 and interleukin-34-derived macrophages possess disparate pathogen recognition capacities. Dev. Comp. Immunol. 2019;98:89–97. doi: 10.1016/j.dci.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 147.Baghdadi M, et al. A role for IL-34 in osteolytic disease of multiple myeloma. Blood Adv. 2019;3:541–551. doi: 10.1182/bloodadvances.2018020008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cheng X, Wan QL, Li ZB. AG490 suppresses interleukin-34-mediated osteoclastogenesis in mice bone marrow macrophages. Cell Biol. Int. 2017;41:659–668. doi: 10.1002/cbin.10771. [DOI] [PubMed] [Google Scholar]
- 149.Udomsinprasert W, Jittikoon J, Honsawek S. Interleukin-34 as a promising clinical biomarker and therapeutic target for inflammatory arthritis. Cytokine Growth Factor Rev. 2019;47:43–53. doi: 10.1016/j.cytogfr.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 150.Li N, et al. The correlation between interleukin-34 and bone erosion under ultrasound in rheumatoid arthritis. Mod. Rheumatol. 2020;30:269–275. doi: 10.1080/14397595.2019.1593576. [DOI] [PubMed] [Google Scholar]
- 151.Li J, et al. New interleukins in psoriasis and psoriatic arthritis patients: the possible roles of interleukin-33 to interleukin-38 in disease activities and bone erosions. Dermatology. 2017;233:37–46. doi: 10.1159/000471798. [DOI] [PubMed] [Google Scholar]
- 152.Wang B, et al. IL-34 upregulated Th17 production through increased IL-6 expression by rheumatoid fibroblast-like synoviocytes. Mediators Inflamm. 2017;2017:1567120. doi: 10.1155/2017/1567120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Batra P, Das S, Patel P. Comparative evaluation of gingival crevicular fluid (GCF) levels of Interleukin-34 levels in periodontally healthy and in patients with chronic and aggressive periodontitis—a cross-sectional study. Saudi Dent. J. 2019;31:316–321. doi: 10.1016/j.sdentj.2019.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang SL, Zhang R, Hu KZ, Li MQ, Li ZC. Interleukin-34 synovial fluid was associated with knee osteoarthritis severity: a cross-sectional study in knee osteoarthritis patients in different radiographic stages. Dis. Markers. 2018;2018:2095480. doi: 10.1155/2018/2095480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang YQ, Cao WJ, Gao YF, Ye J, Zou GZ. Serum interleukin-34 level can be an indicator of liver fibrosis in patients with chronic hepatitis B virus infection. World J. Gastroenterol. 2018;24:1312–1320. doi: 10.3748/wjg.v24.i12.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Liu Q, et al. IL-34 promotes foam cell formation by enhancing CD36 expression through p38 MAPK pathway. Sci. Rep. 2018;8:17347. doi: 10.1038/s41598-018-35485-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang S, et al. IL-34 expression is reduced in hashimoto’s thyroiditis and associated with thyrocyte apoptosis. Front. Endocrinol. (Lausanne) 2018;9:629. doi: 10.3389/fendo.2018.00629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Cheng Y, Yang X, Zhang X, An Z. Analysis of expression levels of IL-17 and IL-34 and influencing factors for prognosis in patients with lupus nephritis. Exp. Ther. Med. 2019;17:2279–2283. doi: 10.3892/etm.2019.7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wada Y, et al. IL-34-dependent intrarenal and systemic mechanisms promote lupus nephritis in MRL-Fas(lpr) mice. J. Am. Soc. Nephrol. 2019;30:244–259. doi: 10.1681/ASN.2018090901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mao YJ, Qin S, Jiao ZF. Wnt pathway regulates IL-34 level in lupus nephritis. Eur. Rev. Med. Pharm. Sci. 2019;23:5360–5365. doi: 10.26355/eurrev_201906_18203. [DOI] [PubMed] [Google Scholar]
- 161.Hamilton JA, Cook AD, Tak PP. Anti-colony-stimulating factor therapies for inflammatory and autoimmune diseases. Nat. Rev. Drug Disco. 2016;16:53–70. doi: 10.1038/nrd.2016.231. [DOI] [PubMed] [Google Scholar]
- 162.Ghia JE, et al. Role of M-CSF-dependent macrophages in colitis is driven by the nature of the inflammatory stimulus. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;294:G770–G777. doi: 10.1152/ajpgi.00453.2007. [DOI] [PubMed] [Google Scholar]
- 163.Segawa M, et al. Suppression of macrophage functions impairs skeletal muscle regeneration with severe fibrosis. Exp. Cell Res. 2008;314:3232–3244. doi: 10.1016/j.yexcr.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 164.Ohno H, et al. The orally-active and selective c-Fms tyrosine kinase inhibitor Ki20227 inhibits disease progression in a collagen-induced arthritis mouse model. Eur. J. Immunol. 2008;38:283–291. doi: 10.1002/eji.200737199. [DOI] [PubMed] [Google Scholar]
- 165.Conway JG, et al. Effects of the cFMS kinase inhibitor 5-(3-methoxy-4-((4-methoxybenzyl)oxy)benzyl)pyrimidine-2,4-diamine (GW2580) in normal and arthritic rats. J. Pharm. Exp. Ther. 2008;326:41–50. doi: 10.1124/jpet.107.129429. [DOI] [PubMed] [Google Scholar]
- 166.Garcia S, et al. Colony-stimulating factor (CSF) 1 receptor blockade reduces inflammation in human and murine models of rheumatoid arthritis. Arthritis Res. Ther. 2016;18:75. doi: 10.1186/s13075-016-0973-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Toh ML, et al. Bone- and cartilage-protective effects of a monoclonal antibody against colony-stimulating factor 1 receptor in experimental arthritis. Arthritis Rheumatol. 2014;66:2989–3000. doi: 10.1002/art.38624. [DOI] [PubMed] [Google Scholar]
- 168.Paniagua RT, et al. Selective tyrosine kinase inhibition by imatinib mesylate for the treatment of autoimmune arthritis. J. Clin. Invest. 2006;116:2633–2642. doi: 10.1172/JCI28546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Dewar AL, et al. Macrophage colony-stimulating factor receptor c-fms is a novel target of imatinib. Blood. 2005;105:3127–3132. doi: 10.1182/blood-2004-10-3967. [DOI] [PubMed] [Google Scholar]
- 170.Hu X, et al. Imatinib inhibits CSF1R that stimulates proliferation of rheumatoid arthritis fibroblast-like synoviocytes. Clin. Exp. Immunol. 2019;195:237–250. doi: 10.1111/cei.13220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Koyama K, et al. Imatinib mesylate both prevents and treats the arthritis induced by type II collagen antibody in mice. Mod. Rheumatol. 2007;17:306–310. doi: 10.1007/s10165-007-0592-9. [DOI] [PubMed] [Google Scholar]
- 172.Wang XF, et al. Colony-stimulating factor 1 receptor inhibition prevents against lipopolysaccharide -induced osteoporosis by inhibiting osteoclast formation. Biomed. Pharmacother. 2019;115:108916. doi: 10.1016/j.biopha.2019.108916. [DOI] [PubMed] [Google Scholar]
- 173.Qi J, et al. Establishment of an orthodontic retention mouse model and the effect of anti-c-Fms antibody on orthodontic relapse. PLoS ONE. 2019;14:e0214260. doi: 10.1371/journal.pone.0214260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Genovese MC, et al. Results from a phase IIA parallel group study of JNJ-40346527, an oral CSF-1R inhibitor, in patients with active rheumatoid arthritis despite disease-modifying antirheumatic drug therapy. J. Rheumatol. 2015;42:1752–1760. doi: 10.3899/jrheum.141580. [DOI] [PubMed] [Google Scholar]
- 175.Lee, K. et al. Blocking of the ubiquitin-proteasome system prevents inflammation-induced bone loss by accelerating M-CSF receptor c-Fms degradation in osteoclast differentiation. Int. J. Mol. Sci.18, 10.3390/ijms18102054 (2017). [DOI] [PMC free article] [PubMed]
- 176.Zaiss MM, et al. Binding immunoglobulin protein (BIP) inhibits tnf-alpha-induced osteoclast differentiation and systemic bone loss in an erosive arthritis model. ACR Open Rheumatol. 2019;1:382–393. doi: 10.1002/acr2.11060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sauter KA, et al. Pleiotropic effects of extended blockade of CSF1R signaling in adult mice. J. Leukoc. Biol. 2014;96:265–274. doi: 10.1189/jlb.2A0114-006R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hashimoto D, et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38:792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Cenci S, Weitzmann MN, Gentile MA, Aisa MC, Pacifici R. M-CSF neutralization and egr-1 deficiency prevent ovariectomy-induced bone loss. J. Clin. Invest. 2000;105:1279–1287. doi: 10.1172/JCI8672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yao GQ, Troiano N, Simpson CA, Insogna KL. Selective deletion of the soluble colony-stimulating factor 1 isoform in vivo prevents estrogen-deficiency bone loss in mice. Bone Res. 2017;5:17022. doi: 10.1038/boneres.2017.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zur Y, et al. A dual-specific macrophage colony-stimulating factor antagonist of c-FMS and alphavbeta3 integrin for osteoporosis therapy. PLoS Biol. 2018;16:e2002979. doi: 10.1371/journal.pbio.2002979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hung JY, et al. Colony-stimulating factor 1 potentiates lung cancer bone metastasis. Lab Invest. 2014;94:371–381. doi: 10.1038/labinvest.2014.1. [DOI] [PubMed] [Google Scholar]
- 183.Wang W, et al. Lymphatic endothelial cells produce M-CSF, causing massive bone loss in mice. J. Bone Min. Res. 2017;32:939–950. doi: 10.1002/jbmr.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ransohoff RM, El Khoury J. Microglia in health and disease. Cold Spring Harb. Perspect. Biol. 2015;8:a020560. doi: 10.1101/cshperspect.a020560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Elmore MR, et al. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron. 2014;82:380–397. doi: 10.1016/j.neuron.2014.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Mancuso R, et al. CSF1R inhibitor JNJ-40346527 attenuates microglial proliferation and neurodegeneration in P301S mice. Brain. 2019;142:3243–3264. doi: 10.1093/brain/awz241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Rosin JM, Vora SR, Kurrasch DM. Depletion of embryonic microglia using the CSF1R inhibitor PLX5622 has adverse sex-specific effects on mice, including accelerated weight gain, hyperactivity and anxiolytic-like behaviour. Brain Behav. Immun. 2018;73:682–697. doi: 10.1016/j.bbi.2018.07.023. [DOI] [PubMed] [Google Scholar]
- 188.Liu Y, et al. Concentration-dependent effects of CSF1R inhibitors on oligodendrocyte progenitor cells ex vivo and in vivo. Exp. Neurol. 2019;318:32–41. doi: 10.1016/j.expneurol.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Schwarzer P, Kokona D, Ebneter A, Zinkernagel MS. Effect of inhibition of colony-stimulating factor 1 receptor on choroidal neovascularization in mice. Am. J. Pathol. 2020;190:412–425. doi: 10.1016/j.ajpath.2019.10.011. [DOI] [PubMed] [Google Scholar]
- 190.Gerber YN, et al. CSF1R inhibition reduces microglia proliferation, promotes tissue preservation and improves motor recovery after spinal cord injury. Front Cell Neurosci. 2018;12:368. doi: 10.3389/fncel.2018.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lee S, Shi XQ, Fan A, West B, Zhang J. Targeting macrophage and microglia activation with colony stimulating factor 1 receptor inhibitor is an effective strategy to treat injury-triggered neuropathic pain. Mol. Pain. 2018;14:1744806918764979. doi: 10.1177/1744806918764979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Neal ML, et al. Pharmacological inhibition of CSF1R by GW2580 reduces microglial proliferation and is protective against neuroinflammation and dopaminergic neurodegeneration. FASEB J. 2020;34:1679–1694. doi: 10.1096/fj.201900567RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Sosna J, et al. Early long-term administration of the CSF1R inhibitor PLX3397 ablates microglia and reduces accumulation of intraneuronal amyloid, neuritic plaque deposition and pre-fibrillar oligomers in 5XFAD mouse model of Alzheimer’s disease. Mol. Neurodegener. 2018;13:11. doi: 10.1186/s13024-018-0244-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Spangenberg E, et al. Sustained microglial depletion with CSF1R inhibitor impairs parenchymal plaque development in an Alzheimer’s disease model. Nat. Commun. 2019;10:3758. doi: 10.1038/s41467-019-11674-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Oh, S. J. et al. Evaluation of the neuroprotective effect of microglial depletion by CSF-1R inhibition in a Parkinson’s animal model. Mol. Imaging Biol.10.1007/s11307-020-01485-w (2020). [DOI] [PubMed]
- 196.Nissen JC, Thompson KK, West BL, Tsirka SE. Csf1R inhibition attenuates experimental autoimmune encephalomyelitis and promotes recovery. Exp. Neurol. 2018;307:24–36. doi: 10.1016/j.expneurol.2018.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Beckmann N, et al. Brain region-specific enhancement of remyelination and prevention of demyelination by the CSF1R kinase inhibitor BLZ945. Acta Neuropathol. Commun. 2018;6:9. doi: 10.1186/s40478-018-0510-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Srivastava PK, et al. A systems-level framework for drug discovery identifies Csf1R as an anti-epileptic drug target. Nat. Commun. 2018;9:3561. doi: 10.1038/s41467-018-06008-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Edginton-White B, et al. Global long terminal repeat activation participates in establishing the unique gene expression programme of classical Hodgkin lymphoma. Leukemia. 2019;33:1463–1474. doi: 10.1038/s41375-018-0311-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J. Exp. Med. 2001;193:727–740. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Pedersen MB, et al. High intratumoral macrophage content is an adverse prognostic feature in anaplastic large cell lymphoma. Histopathology. 2014;65:490–500. doi: 10.1111/his.12407. [DOI] [PubMed] [Google Scholar]
- 202.Zhang QW, et al. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS ONE. 2012;7:e50946. doi: 10.1371/journal.pone.0050946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Akazawa Y, et al. M-CSF receptor antagonists inhibit the initiation and progression of hepatocellular carcinoma in mice. Anticancer Res. 2019;39:4787–4794. doi: 10.21873/anticanres.13663. [DOI] [PubMed] [Google Scholar]
- 204.DeNardo DG, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Disco. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Komohara Y, et al. Potential anti-lymphoma effect of M-CSFR inhibitor in adult T-cell leukemia/lymphoma. J. Clin. Exp. Hematop. 2018;58:152–160. doi: 10.3960/jslrt.18034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zhu HY, et al. In silico identification and characterization of N-Terminal acetyltransferase genes of poplar (Populus trichocarpa) Int. J. Mol. Sci. 2014;15:1852–1864. doi: 10.3390/ijms15021852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Pyonteck SM, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat. Med. 2013;19:1264–1272. doi: 10.1038/nm.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Strachan DC, et al. CSF1R inhibition delays cervical and mammary tumor growth in murine models by attenuating the turnover of tumor-associated macrophages and enhancing infiltration by CD8(+) T cells. Oncoimmunology. 2013;2:e26968. doi: 10.4161/onci.26968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Meziani, L. et al. CSF1R inhibition prevents radiation pulmonary fibrosis by depletion of interstitial macrophages. Eur. Respir. J.51, 10.1183/13993003.02120-2017 (2018). [DOI] [PubMed]
- 210.Edwards DKT, et al. CSF1R inhibitors exhibit antitumor activity in acute myeloid leukemia by blocking paracrine signals from support cells. Blood. 2019;133:588–599. doi: 10.1182/blood-2018-03-838946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Papin A, et al. CSF1R and BTK inhibitions as novel strategies to disrupt the dialog between mantle cell lymphoma and macrophages. Leukemia. 2019;33:2442–2453. doi: 10.1038/s41375-019-0463-3. [DOI] [PubMed] [Google Scholar]
- 212.Edwards VD, et al. Targeting of colony-stimulating factor 1 receptor (CSF1R) in the CLL microenvironment yields antineoplastic activity in primary patient samples. Oncotarget. 2018;9:24576–24589. doi: 10.18632/oncotarget.25191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Wang Q, et al. Therapeutic effects of CSF1R-blocking antibodies in multiple myeloma. Leukemia. 2018;32:176–183. doi: 10.1038/leu.2017.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Zhang L, et al. Single-cell analyses inform mechanisms of myeloid-targeted therapies in colon. Cancer Cell. 2020;181:442–459 e429. doi: 10.1016/j.cell.2020.03.048. [DOI] [PubMed] [Google Scholar]
- 215.Kumar V, et al. Cancer-associated fibroblasts neutralize the anti-tumor effect of CSF1 receptor blockade by inducing PMN-MDSC infiltration of tumors. Cancer Cell. 2017;32:654–668 e655. doi: 10.1016/j.ccell.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Swierczak A, et al. The promotion of breast cancer metastasis caused by inhibition of CSF-1R/CSF-1 signaling is blocked by targeting the G-CSF receptor. Cancer Immunol. Res. 2014;2:765–776. doi: 10.1158/2326-6066.CIR-13-0190. [DOI] [PubMed] [Google Scholar]
- 217.Hiraga T, Nakamura H. Imatinib mesylate suppresses bone metastases of breast cancer by inhibiting osteoclasts through the blockade of c-Fms signals. Int. J. Cancer. 2009;124:215–222. doi: 10.1002/ijc.23903. [DOI] [PubMed] [Google Scholar]
- 218.Fend L, et al. Therapeutic effects of anti-CD115 monoclonal antibody in mouse cancer models through dual inhibition of tumor-associated macrophages and osteoclasts. PLoS ONE. 2013;8:e73310. doi: 10.1371/journal.pone.0073310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ramesh A, Kumar S, Nandi D, Kulkarni A. CSF1R- and SHP2-inhibitor-loaded nanoparticles enhance cytotoxic activity and phagocytosis in tumor-associated macrophages. Adv. Mater. 2019;31:e1904364. doi: 10.1002/adma.201904364. [DOI] [PubMed] [Google Scholar]
- 220.Ramesh A, Brouillard A, Kumar S, Nandi D, Kulkarni A. Dual inhibition of CSF1R and MAPK pathways using supramolecular nanoparticles enhances macrophage immunotherapy. Biomaterials. 2020;227:119559. doi: 10.1016/j.biomaterials.2019.119559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Cannarile MA, et al. Colony-stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. J. Immunother. Cancer. 2017;5:53. doi: 10.1186/s40425-017-0257-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Papadopoulos KP, et al. First-in-human study of AMG 820, a monoclonal anti-colony-stimulating factor 1 receptor antibody, in patients with advanced solid tumors. Clin. Cancer Res. 2017;23:5703–5710. doi: 10.1158/1078-0432.CCR-16-3261. [DOI] [PubMed] [Google Scholar]
- 223.von Tresckow B, et al. An open-label, multicenter, phase I/II study of JNJ-40346527, a CSF-1R inhibitor, in patients with relapsed or refractory Hodgkin lymphoma. Clin. Cancer Res. 2015;21:1843–1850. doi: 10.1158/1078-0432.CCR-14-1845. [DOI] [PubMed] [Google Scholar]
- 224.Butowski N, et al. Orally administered colony stimulating factor 1 receptor inhibitor PLX3397 in recurrent glioblastoma: an Ivy Foundation Early Phase Clinical Trials Consortium phase II study. Neuro. Oncol. 2016;18:557–564. doi: 10.1093/neuonc/nov245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Deng M, et al. Clinical efficacy and safety of imatinib treatment in children and adolescents with chronic myeloid leukemia: A single-center experience in China. Medicine. 2020;99:e19150. doi: 10.1097/MD.0000000000019150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Tap WD, et al. Structure-guided blockade of CSF1R kinase in tenosynovial giant-cell tumor. N. Engl. J. Med. 2015;373:428–437. doi: 10.1056/NEJMoa1411366. [DOI] [PubMed] [Google Scholar]
- 227.Cassier PA, et al. CSF1R inhibition with emactuzumab in locally advanced diffuse-type tenosynovial giant cell tumours of the soft tissue: a dose-escalation and dose-expansion phase 1 study. Lancet Oncol. 2015;16:949–956. doi: 10.1016/S1470-2045(15)00132-1. [DOI] [PubMed] [Google Scholar]
- 228.Bendell Johanna C. et al. Abstract A252: a phase 1 study of ARRY-382, an oral inhibitor of colony-stimulating factor-1 receptor (CSF1R), in patients with advanced or metastatic cancers. Mol. Cancer Ther.12 (2013).
- 229.Sadis S, et al. Safety, pharmacokinetics, and pharmacodynamics of PD-0360324, a human monoclonal antibody to monocyte/macrophage colony stimulating factor, in healthy volunteers. Arthritis Rheum. 2009;60:408. [Google Scholar]
- 230.Rodriguez RM, et al. Signal integration and transcriptional regulation of the inflammatory response mediated by the GM-/M-CSF signaling axis in human monocytes. Cell Rep. 2019;29:860–872 e865. doi: 10.1016/j.celrep.2019.09.035. [DOI] [PubMed] [Google Scholar]