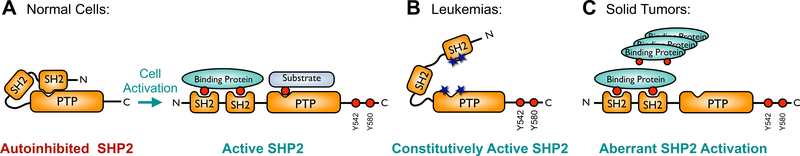
Figure 1. Regulation of SHP2 activity.
(A) SHP2 activity is tightly regulated in normal cells. Under basal conditions, its N-terminal SH2 domain occludes the active site within the phosphatase (PTP) domain, resulting in autoinhibition. RTK growth factor and cytokine stimulation leads to tyrosine phosphorylation of adapter proteins, which then bind to the SH2 domains, causing a conformational change whereby the SHP2 active site becomes accessible to its substrates. (B) Somatic SHP2 mutations, frequently found in leukemias, are located at the interface between the N-SH2 and phosphatase domains and prevent SHP2 from adopting the closed, autoinhibited conformation, resulting in a constitutively active phosphatase. (C) In solid tumors, amplification or overexpression of growth factors, RTKs, or scaffolding adapters result in aberrant activation of SHP2.