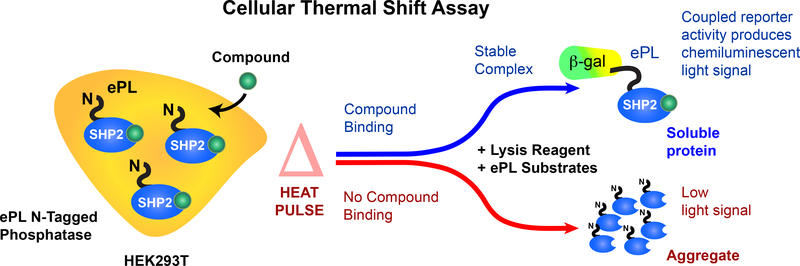
Figure 2. SHP2 cellular thermal shift principles.
Our cellular target engagement assay is based on a β-galactosidase (β-gal) enzyme fragment complementation (EFC) assay. Cells (HEK293T), expressing full-length SHP2 with an ePL fusion tag (42 amino acid fragment of β-gal), are treated with a probe compound and are subjected to a short heat pulse that causes SHP2 to denature and form inaccessible insoluble aggregates, rendering the ePL tag inaccessible. Specific binding of the probe compound to SHP2 can enhance its thermal stability. Thermal stabilization provides more soluble target protein with the ePL tag for complementation in the reporter enzyme chemiluminescence system. Aggregates result in reduced complementation and luminescence. (This figure has been modified from Ref. 36)