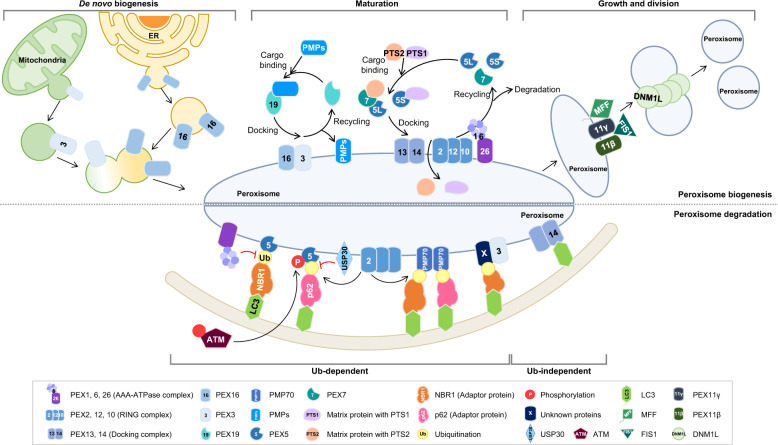
Fig. 1. Schematics of the peroxisome biogenesis and degradation systems.
The number of peroxisomes is regulated by de novo biogenesis and the growth and division of pre-existing organelles. First, peroxisomes can be formed by the maturation of preperoxisomal vesicles that emerge from the ER or mitochondria and contain peroxisomal membrane proteins, including PEX16, PEX3, and PEX14. Preperoxisomal vesicle fusion results in the generation of mature peroxisomes mediated by PEX19. Second, peroxisomes can be formed by the elongation and division of mature peroxisomes, which are cleaved by the proteins PEX11, Fis1, MFF, and DNM1L (top). Pexophagy is regulated by ubiquitination-dependent and ubiquitination-independent pathways. The ubiquitination of the cytosolic region of peroxisomes triggers their degradation by pexophagy. During oxidative stress, ATM interacts with and phosphorylates PEX5, which promotes PEX5 ubiquitination by PEX2. Ubiquitinated PEX5 is recognized by p62, which recruits the autophagosome. The peroxisomal AAA-type ATPase complex, PEX1, PEX6, and PEX26, prevents pexophagy by regulating the accumulation of ubiquitinated PEX5. During amino acid starvation conditions, PEX2 regulates the ubiquitination of PEX5 and PMP70, increasing pexophagy in an NBR1-dependent manner. USP30 prevents pexophagy by counteracting PEX2. In contrast, PEX14 directly interacts with LC3 under nutrient deprivation conditions (bottom).