Figure 2.
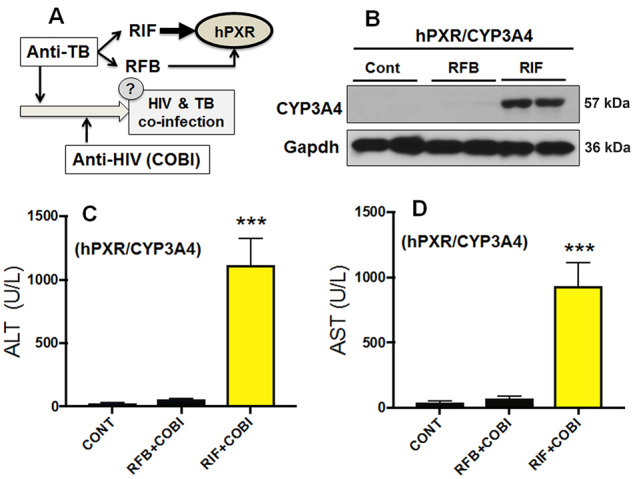
RFB, a PXR-neutral analog of RIF, does not potentiate COBI hepatotoxicity in hPXR/CYP3A4 mice. A, A scheme showing the WHO guideline for the treatment of HIV and TB co-infection, in which lead-in treatment with anti-TB drugs is recommended. Both RIF and RFB are commonly used anti-TB drugs that can interact with human PXR (hPXR). We therefore tested whether lead-in treatment with RFB will potentiate COBI hepatotoxicity through hPXR. B, CYP3A4 induction in the liver of hPXR/CYP3A4 mice pretreated with RIF or RFB. Expression of CYP3A4 was analyzed by Western blotting. Gapdh was used as a loading control. C, D, Serum activities of ALT and AST in hPXR/CYP3A4 mice pretreated with RIF or RFB followed by COBI. All data are shown as mean ± SEM (n = 3–7). Statistical significance was determined by one-way ANOVA with Tukey’s post hoc test. ***p < .001.
