Figure 2.
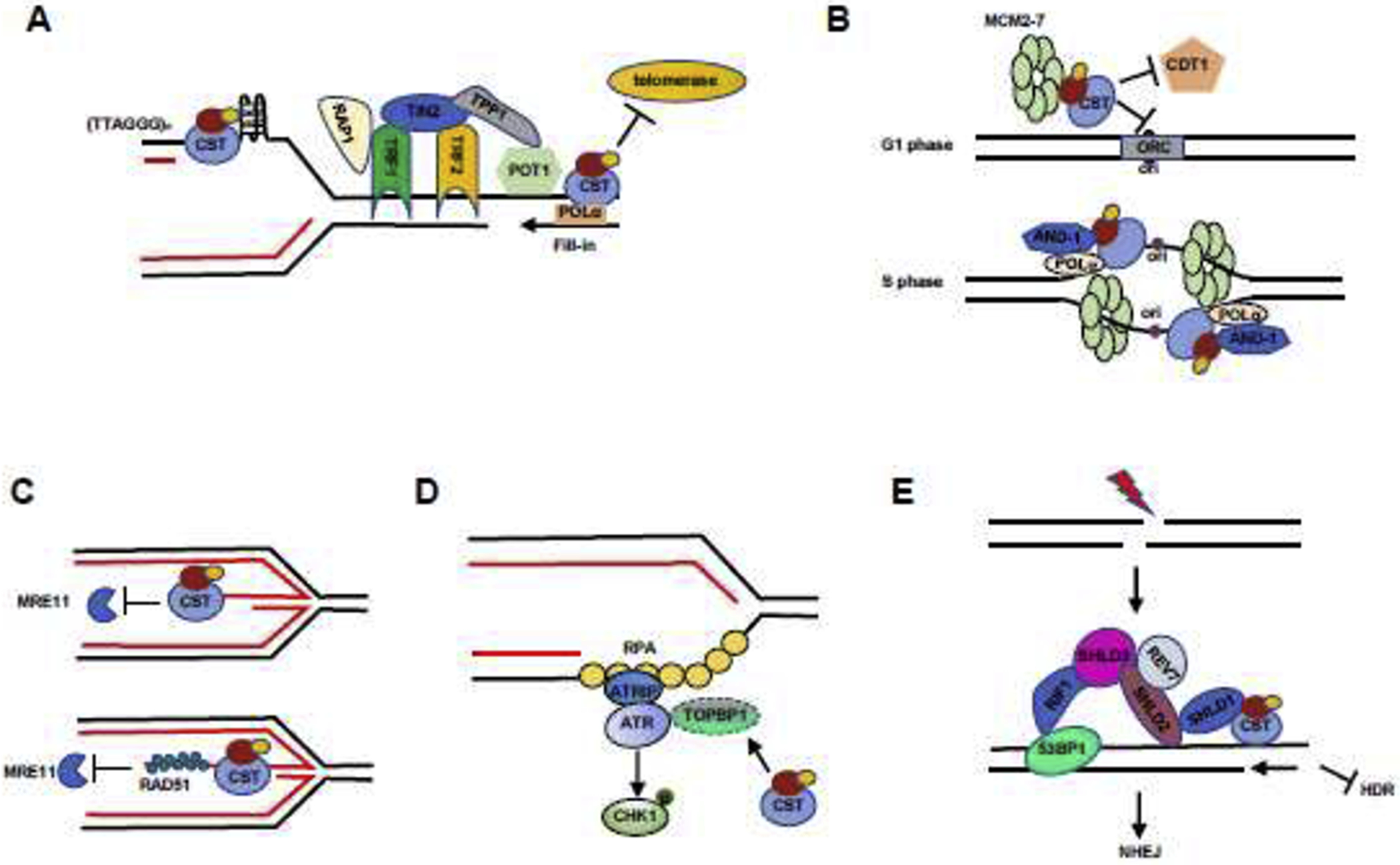
Models illustrating the roles of CST in genome stability maintenance.
(A) CST regulates telomere stability via the telomeric synthesis processes. In the ds telomeric region, CST resolves G4 structures and relieves replication stress in the telomeric region. At telomere ends, CST binds to ss G-overhangs to inhibit the access of telomerase to telomeres. CST also mediates C-strand fill-in to replenish C-strands.
(B) CST regulates DNA replication initiation under normal replication condition. In the G1 phase, CST blocks origin licensing by interacting with MCM to prevent CDT1 interacting with MCM. In the S phase, CST interacts with AND-1 and POLα and then facilitates replisome assembly and subsequent initiation of DNA synthesis.
(C) CST protects reversed fork stability under replication stress. Top: CST directly binds to the regressed arms of reversed forks to inhibit unscheduled MRE11 degradation of nascent strand DNA. In addition, CST indirectly protects reversed forks via recruiting RAD51 (bottom).
(D) CST regulates the ATR-CHK1 pathway under replication stress. CST prevents the degradation of the ATR activator TOPBP1. CST deficiency decreases TOPBP1 protein level, therefore suppressing CHK1 phosphorylation following replication stress.
(E) CST controls end-resection during DSB repair and favors c-NHEJ. At DSB sites, 53BP1-RIF1 recruits the Shieldin complex (SHLD1-SHLD2-SHLD3-REV7), which interacts with and recruits CST to DSB ends to counteract end resection.
