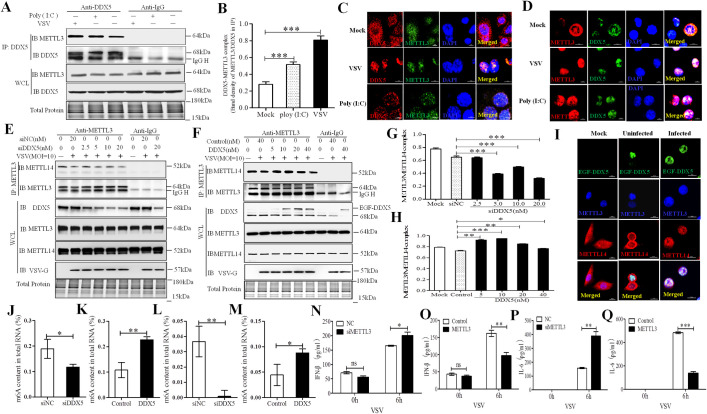
Fig 3. DDX5 regulated the m6A writer complex by recruiting METTL3 after VSV infection.
(A, B): VSV promoted the interaction of DDX5 and METTL3 in MEFs. MEFs were infected with VSV or treated with Poly (I:C) for 8h and lysed with NP40 lysis buffer. Lysates were subjected to IP with DDX5 antibody or IgG. Whole-cell and IP lysates were analyzed by western blotting. The DDX5-METTL3 interaction complex was quantified by the western blot band density of METTL3/DDX5 of IP lysates. (C, D): Co-localization of DDX5 and METTL3 in MEFs (C) or macrophages (D) after VSV infection. MEFs/macrophages were cultured in slices and transfected with VSV or treated with Poly (I:C) for 8h. Slices were fixed, permeabilized, and incubated with rabbit anti-DDX5 and mouse anti-METTL3 antibodies followed by Alexa Fluor 546 labeled goat anti-rabbit IgG and Alexa Fluor 488 labeled goat anti-mouse IgG. Nuclei were stained with DAPI. Cells were observed by LSCM. Scale bars, 10 μm. (E, F): The interaction of METTL3 and METTL14 in DDX5-knockdown MEFs (E) or enhanced green fluorescent (EGF) tag fused DDX5-overexpressed MEFs (F). MEFs were transfected with different doses of DDX5 siRNA (siNC) for 48 h (E) or with different doses of EGF-DDX5 expression plasmids (control vector) for 24 h. Cells were infected with VSV (MOI = 10) for 8 h and lysed with NP40 lysis buffer, lysates were subjected to IP with rabbit METTL3 antibody or IgG. Whole-cell and IP lysates were analyzed by western blotting. (G, H): METTL3-METTL14 complex was quantified based on band intensity on western blot, METTL14 was normalized to METTL3 in IP assay, METTL3-METTL14 complex was determined in DDX5 knockdown IP assay (E) or DDX5 over-expressed IP assay (F). I: Co-localization of exogenous DDX5, METTL3, and METTL14 in VSV-infected MEFs. MEFs were cultured in slices and transfected with EGF-DDX5 expression plasmids. After 24 h, cells were infected with VSV for 8h; then, slices were fixed, permeabilized, and incubated with rabbit anti-METTL3 and mouse anti-METTL14 antibodies followed by goat anti-mouse IgG (H+L) highly cross-adsorbed secondary antibody, Alexa Fluor 647, goat anti-rabbit IgG (H+L) secondary antibody, and DyLight 405. Nuclei were stained with DAPI. Normal MEFs were used as controls. Slices were observed by LSCM. Scale bars, 10 μm. (J, K): The m6A content in total RNA was detected in DDX5-silenced MEFs (J) or DDX5-expressing MEFs (K) after VSV infection by ELISA. MEFs were transfected with DDX5 siRNA for 48h (J) or DDX5 expression plasmid for 24 h (K). Cells were infected with VSV for 8 hours used to detect m6A content in total RNA using anm6A RNA Methylation Assay Kit. (L, M): The m6A content in total RNA was detected in DDX5-silenced macrophages (L) or DDX5-expressing macrophages (M) after VSV infection by ELISA. Macrophages were infected with ADV1-siDDX5 (L) or lentiviral DDX5 for 48 h (M). Cells were infected with VSV for 8h and used to detect the m6A content in total RNA using anm6A RNA Methylation Assay Kit. (N, O): ELISA analysis of IFN-β in the supernatants of METTL3-knockdown or–overexpressed MEFs infected with VSV for 0 or 6 h. (P, Q): ELISA analysis of IL-6 in supernatants of METTL3-knockdown or -overexpressed MEFs infected with VSV for 0 or 6 h. All data are mean ± SEM of biologically independent samples. Data are representative of three independent experiments. ns, no significant difference. *p<0.05, **p<0.01, ***p<0.001 (Student’s t-test).