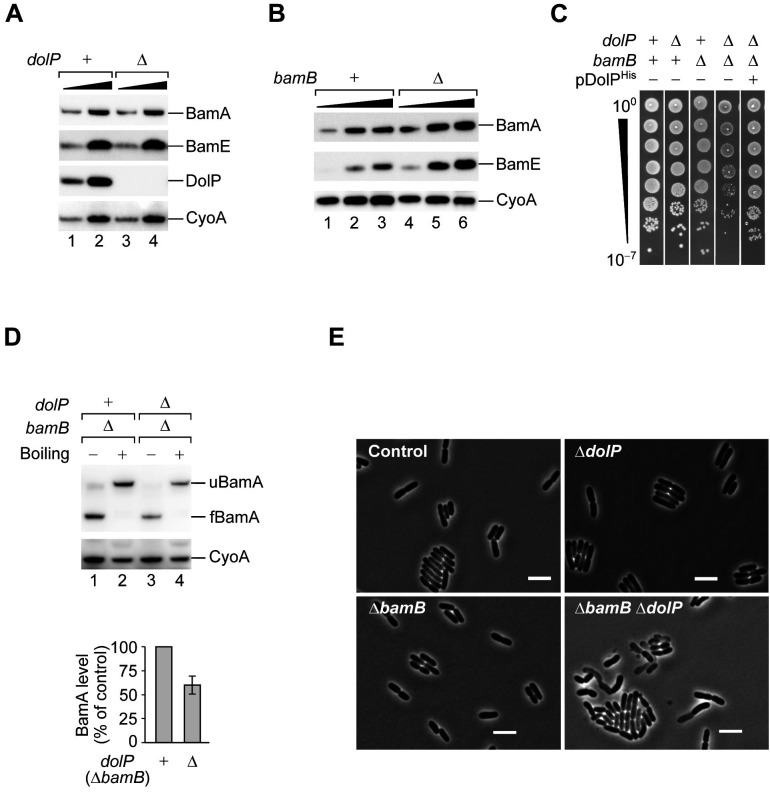
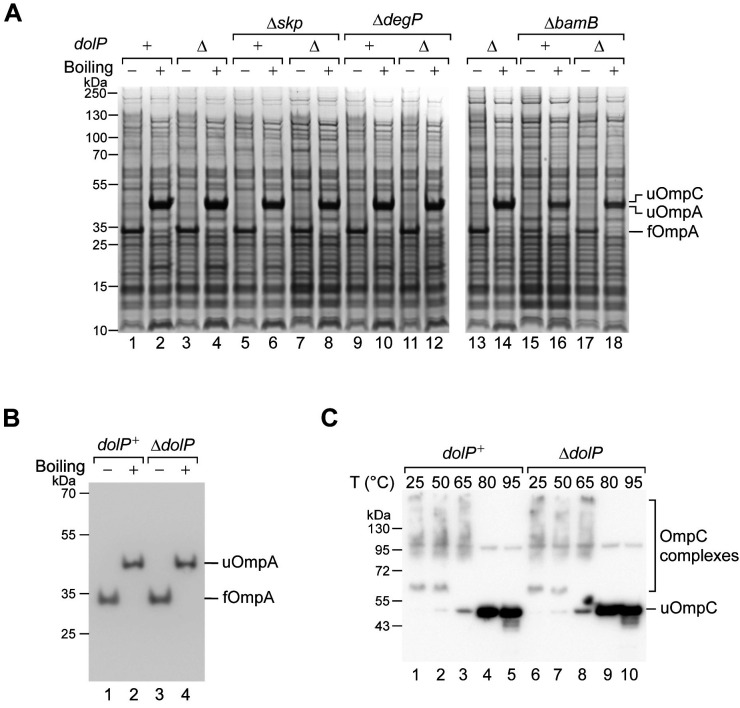
Figure 2. DolP promotes fitness in cells that undergo envelope stress.
(A) One- and three-fold amounts of the total cell lysate fractions obtained from a BW25113 (dolP+) strain and a derivative ΔdolP strain were analysed by SDS-PAGE and immunoblotting using the indicated antisera. (B) One-, two-, and three-fold amounts of the total cell lysate fractions obtained from a BW25113 (bamB+) strain and a derivative ΔbamB strain were analysed by SDS-PAGE and immunoblotting using the indicated antisera. (C) BW25113 and derivative cells deleted of dolP, bamB, or both genes were cultured, serially diluted, and spotted on LB agar. Cells deleted of both dolP and bamB and transformed with pDolPHis were cultured, serially diluted, and spotted on LB agar supplemented with ampicillin. (D) The envelope fractions of the indicated strains were analysed by SDS-PAGE and immunoblotting. Prior to gel loading, samples were incubated at 25°C (Boiling −) or 99°C (Boiling +). The total amounts of BamA in ΔbamB dolP+ and ΔbamB ΔdolP strains were quantified, normalized to the amount of the inner membrane protein CyoA, and expressed as percentage of the value obtained for the ΔbamB dolP+ sample. Data are reported as means ± standard error of the mean (SEM, N = 3). u, unfolded; f, folded. (E) Overnight cultures of BW25113 (control), ΔdolP, ΔbamB, and ΔdolP ΔbamB, were freshly diluted in LB medium and re-incubated at 30°C until OD600 = 0.3. Cells were visualized on 1% (w/v) agarose pads by phase contrast microscopy. Bar = 5 μm.