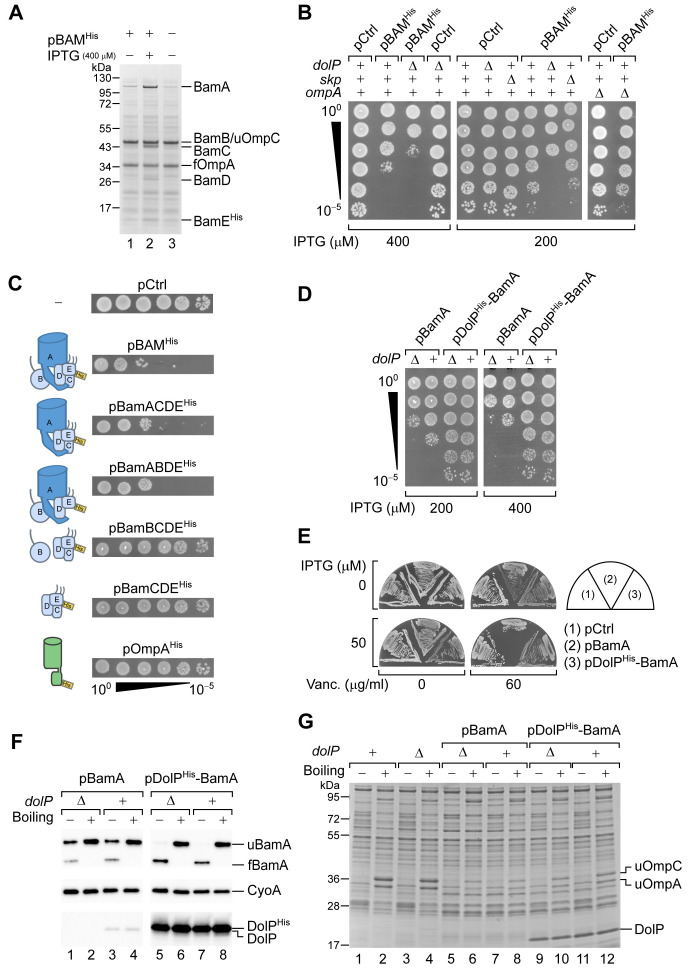
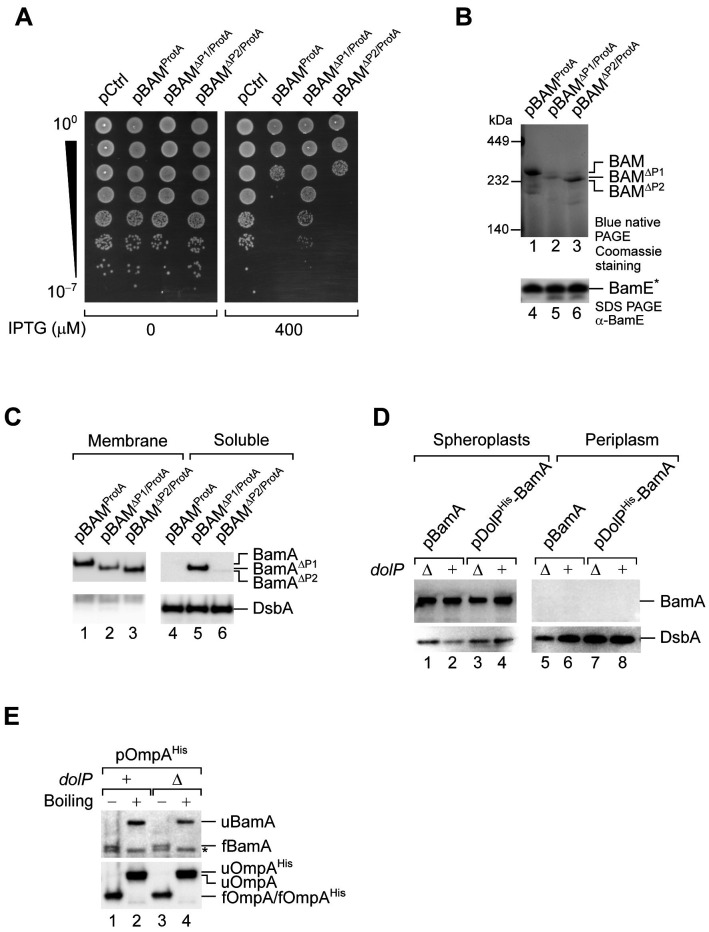
Figure 3. DolP opposes an envelope detrimental effect caused by BamA overaccumulation in the OM.
(A) BW25113 cells harbouring pBAMHis where indicated were cultured and supplemented with no IPTG or 400 μM IPTG for 1 hr prior to collecting cells. The protein contents of the envelope fractions were analysed by SDS-PAGE and coomassie staining. Prior to loading, samples were heated for 5 min at 90°C, a temperature which is not sufficient to fully denature OmpA (folded OmpA, fOmpA). The band of BamB overlaps with the band of the major porin unfolded OmpC (uOmpC). (B) The BW25113 and the derivative ΔdolP, Δskp, or ΔompA strains carrying an empty control vector (pCtrl) or pBAMHis were serially diluted and spotted onto LB agar supplemented with IPTG as indicated. (C) BW25113 cells carrying a control empty vector (pCtrl), or the indicated plasmids for ectopic overproduction of BAM, or subsets of BAM subunits, or OmpAHis were serially diluted and spotted onto LB agar containing 400 μM IPTG. The diagrams depict the overproduced proteins. (D) BW25113 and derivative ΔdolP cells carrying the indicated plasmids for ectopic overproduction of either BamA alone or both DolPHis and BamA were serially diluted and spotted onto LB agar supplemented with IPTG as indicated. (E) BW25113 cells carrying the indicated plasmids were cultured overnight and streaked onto LB agar containing IPTG and vancomycin as indicated. (F) Heat-modifiability of BamA in wild-type and ΔdolP cells carrying the indicated plasmids. When the cultures reached the mid-exponential phase, the expression of BamA was induced for 2 hr with 200 μM IPTG. Total cell proteins were incubated at 25°C (Boiling −) or at 99°C (Boiling +), separated by SDS-PAGE and analysed by immunoblotting using the indicated antisera. u, unfolded; f, folded. (G) Heat modifiability of the protein contents of the envelope fraction of BW25113 (dolP+) or ΔdolP cells carrying no vector or transformed with pBamA or pDolPHis-BamA. Plasmid-borne genes were induced with 200 μM IPTG for 2 hr prior to collecting cells. The envelope fractions were mixed with SDS-PAGE loading buffer, incubated at 25°C (Boiling −) or 99°C (Boiling +) for 10 min, and analysed by SDS-PAGE and coomassie staining. u, unfolded.