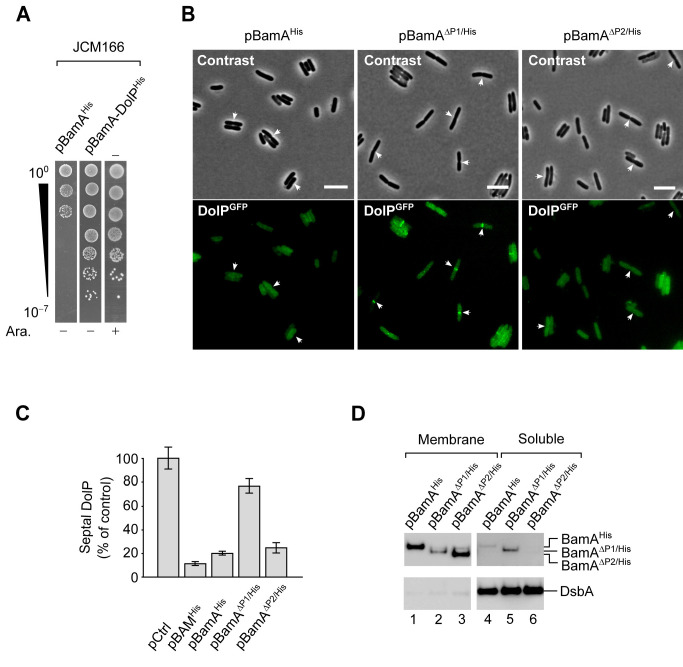
Figure 5. BamA overaccumulation in the OM and envelope stress interfere with the mid-cell localization of DolP.
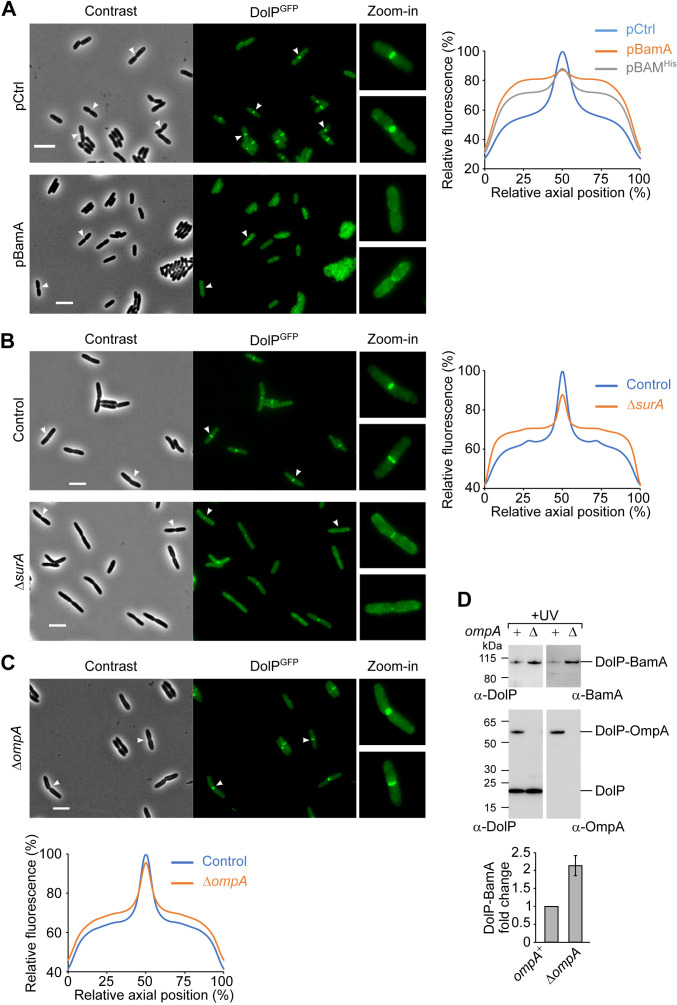
(A) Overnight cultures of BW25113 cells harbouring the chromosomal fusion dolP-gfp and transformed with either pCtrl (empty vector) or pBamA were freshly diluted in minimal M9 medium, incubated at 30°C until OD600 = 0.1 and supplemented with 400 μM IPTG for 1 hr. Cell samples were visualized on 1% (w/v) agarose pads by phase contrast and fluorescence microscopy. Arrowheads indicate envelope constriction sites between forming daughter cells. Bar = 5 μm. The collective profiles of fluorescence distribution versus the relative position along the cell axis were plotted: pCtrl, blue; pBamA, orange; pBAMHis, grey (images of cells transformed with pBAMHis are shown in Figure 5—figure supplement 3A). Only cells with a constriction (N = 361, pCtrl; N = 187, pBamA; N = 187, pBAMHis) were taken into account for the collective profile plots. Fluorescence intensities were normalized to the mid-cell value obtained for the control sample. (B) Overnight cultures of BW25113 (control) or ΔsurA derivative cells carrying the dolP-gfp chromosomal fusion were freshly diluted in LB medium and incubated at 30°C until OD600 = 0.3. Cell samples were visualized as in (A). Bar = 5 μm. The collective profiles of fluorescence distribution versus the relative position along the cell axis is shown for ΔsurA cells (orange) and surA+ control cells (blue). Only cells with a constriction (N = 318, Control; N = 320, ΔsurA) were taken into account for the collective profile plots. Fluorescence intensities were normalized to the mid-cell value obtained for the control sample. (C) Overnight cultures of ΔompA cells carrying the dolP-gfp chromosomal fusion were cultured and visualized as in (B). Bar = 5 μm. The collective profiles of fluorescence distribution versus the relative position along the cell axis is shown for ΔompA cells (orange) and an ompA+ (control) strain that was cultured and visualized in a parallel experiment (blue). Only cells with a constriction (N = 287, Control; N = 193, ΔompA) were taken into account for the collective profile plots. Fluorescence intensities were normalized to the mid-cell value obtained for the control sample. (D) UV photo-crosslinking of ΔdolP and ΔdolP ΔompA cells transformed with pBamA-DolPHis harbouring an amber codon at position V52 of the dolP ORF. Signals obtained with the anti-BamA antiserum were quantified and showed in the histogram. The amount of DolP-BamA crosslink product obtained with samples lacking OmpA is expressed as fold change of the amount of the same product obtained in samples expressing OmpA. Data are reported as mean ± SEM (N = 3).
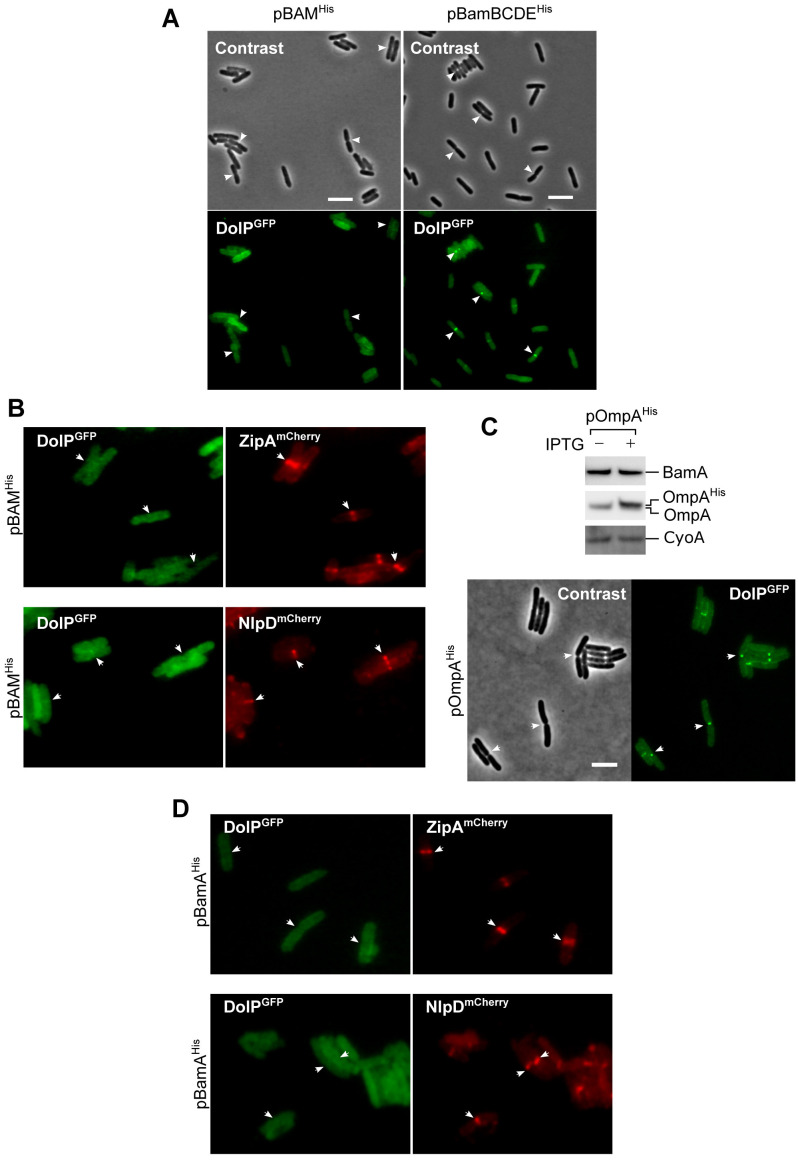
Figure 5—figure supplement 1. Effect of the lack or the overproduction of DolP on BAM localization.
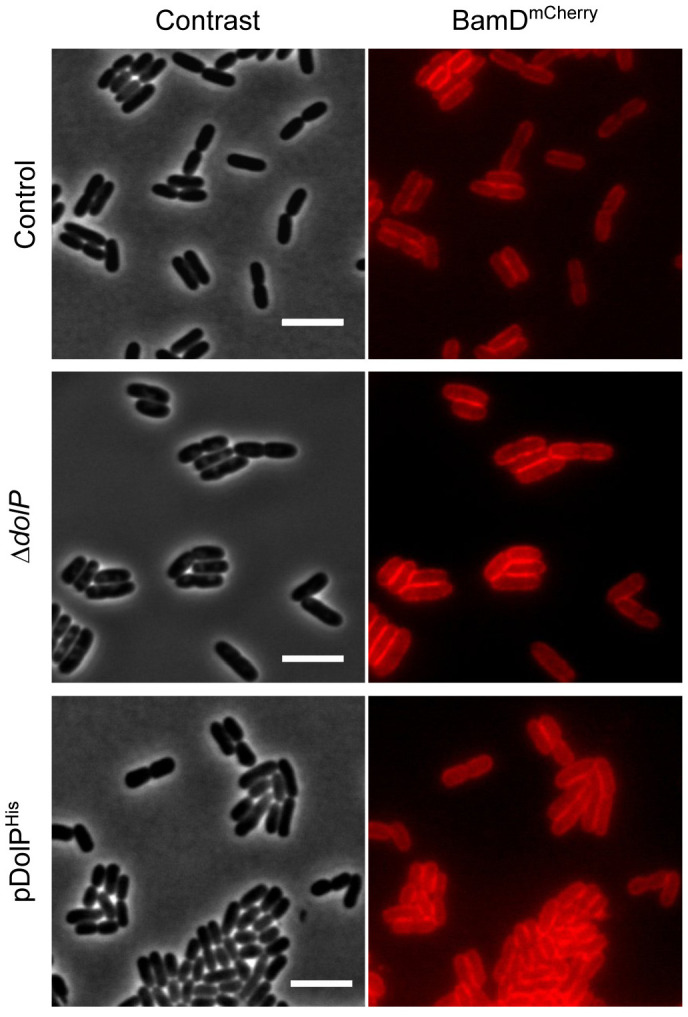
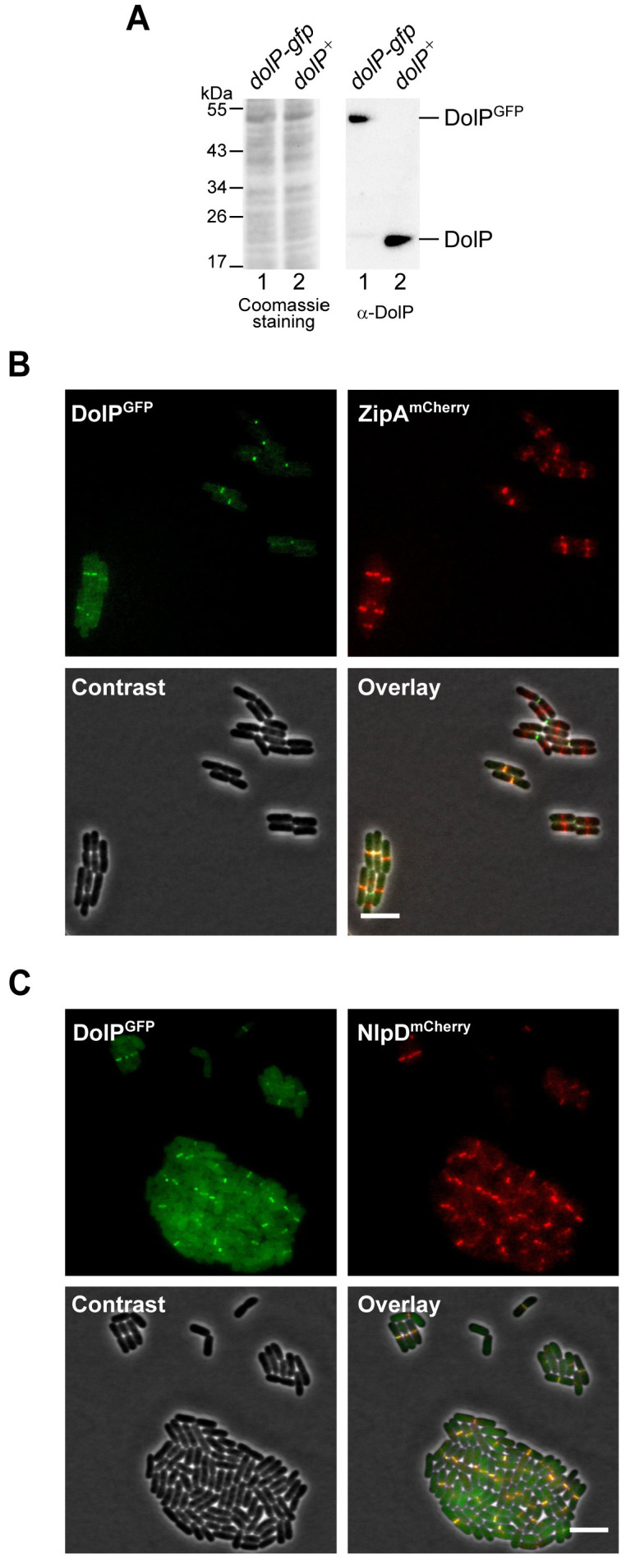
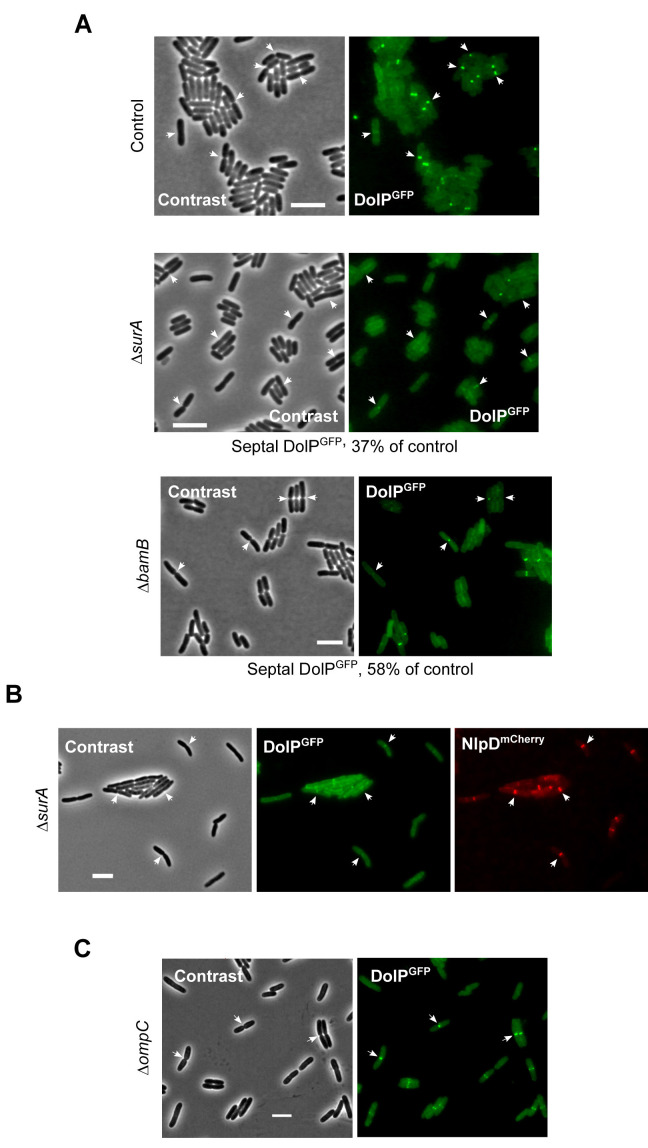
Figure 5—figure supplement 2. DolPGFP, NlpDmCherry, and ZipAmCherry mid-cell localization patterns.