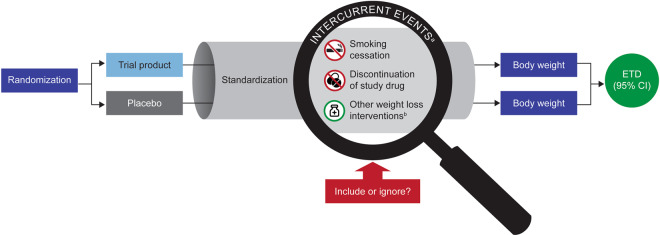
Fig. 1. Intercurrent events and their implications in weight management clinical trials.
CI confidence interval, ETD estimated treatment difference. aIntercurrent events are likely to be unbalanced between treatment arms, potentially introducing bias. bAdopting other weight-loss medication recorded as concomitant medication during the trial. Non-pharmacological measures could also be considered, e.g., actively engaging in additional weight-loss programs such as joining a gym or commercial weight-loss program, or bariatric surgery.