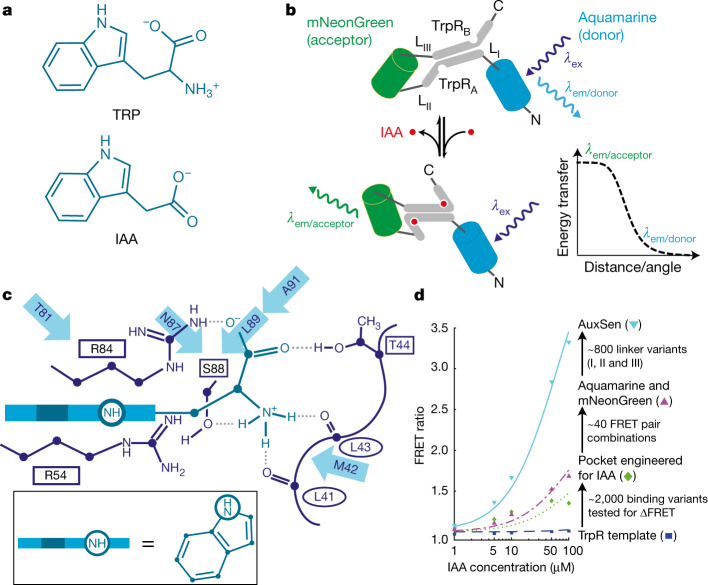
Fig. 1. Summary of the design process.
a, Chemical structures of TRP and IAA. b, Principle of the sensor design. Only in the presence of IAA (red) are the fluorophores (mNeonGreen and Aquamarine) sufficiently close and in the correct orientation for energy transfer (EFRET). N and C represent the N and C termini of the proteins, respectively; L represents the linker; and λex and λem represent the excitation and emission wavelengths, respectively. c, Structure of the binding pocket of TrpR with ligand in side view (boxed) (modified from ref. 8). Interactions with the side chains of R84, S88 and T44 (second TrpR chain) as well as the backbone carbonyl groups of L41 and L43 (second TrpR chain) are shown explicitly. Further residues that were mutated in this study are indicated with arrows. d, Major steps in the design of the sensor (AuxSen), and their cumulative contribution to the change in FRET ratio (ΔFRET) plotted against IAA concentration . Template sensor construct, TrpR–eCFP–Venus (blue squares); engineered binding pocket for IAA, TrpR(M42F/T44L/T81M/N87G/S88Y)–eCFP–Venus (green diamonds); optimized fluorophore combination, TrpR(M42F/T44L/T81M/N87G/S88Y)–mNeonGreen–Aquamarine (purple triangles); AuxSen, TrpR(M42F/T44L/T81M/N87G/S88Y)–mNeonGreen–Aquamarine with optimised linkers I, II and III (light blue inverted triangles).