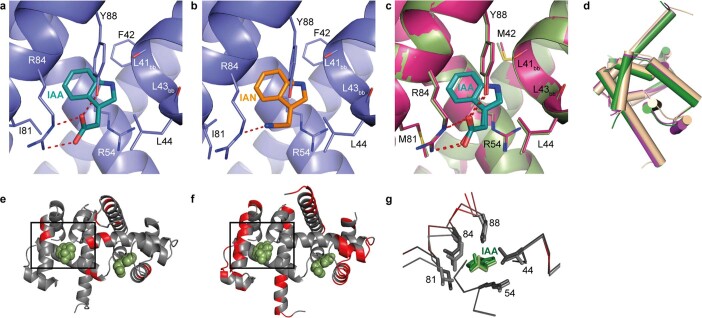
Extended Data Fig. 2. Details observed in the crystal structures.
a, Structure of IAA in the binding pocket of TrpR(M42F/T44L/T81I/S88Y). b, Structure of IAN bound to the same variant as in a. c, Overlay of IAA in TrpR(T44L/T81M/S88Y) (magenta) and TrpR(T44L/T81M/N87G/S88Y) (green). d, Structural overview of variants TrpR(T44L/T81M/N87G/S88Y) (green), TrpR(T44L/T81M/S88Y) (magenta), and TrpR(M42F/T44L/T81M/N87G/S88Y) (AuxSen, gold). It is apparent that AuxSen differs from the two intermediate structures regarding the overall arrangement of the helices. e–g, The structure of TrpR(S88Y/T44L) and all variant structures based on it show a slight relocation of the backbone of residues 70–90. This is probably due to the fact that all structures based on this variant crystallize in the orthorhombic space group P212121 (f) as opposed to the tetragonal space group P43 found for TrpR–IAA and TrpR(S88Y)–IAA (e). Both geometries have been found in earlier crystal structures of TrpR, for example PDB 1ZT9 (tetragonal) and 2OZ9 (orthorhombic). It seems that the introduction of the T44L mutation strongly favours crystallization in the orthorhombic geometry. In the P212121 space group, crystals form more extensive crystal contacts. The structure overlay (g) shows how several residues are displaced (residues that have symmetry mates within 3 Å are shown in red, and the ligand IAA is shown in green). However, interactions and positioning of the ligands are maintained. Nonetheless, we only compare backbone coordinates between variants of the same space group, to exclude misinterpretations due to crystal contacts.