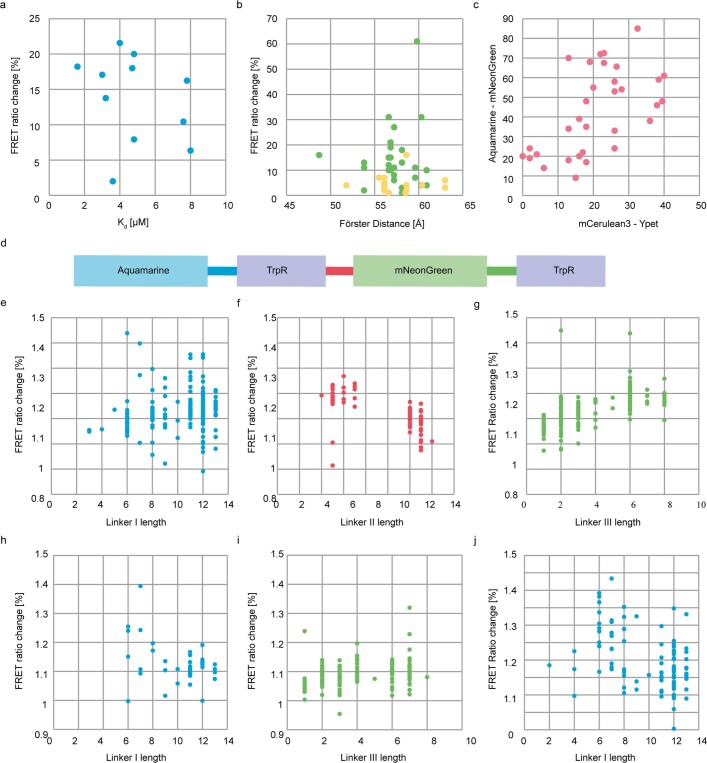
Extended Data Fig. 3. Parameters tested for potential influence on the change of FRET ratio.
a, Change in FRET ratio upon IAA treatment plotted against the dissociation constant (Kd) of the same variant as determined by ITC. b, FRET ratio changes do not correlate with the Förster distance. Blue–yellow pairs are marked in green, yellow–red pairs are in orange. Blue–yellow pairs, in general, show a higher FRET ratio change upon IAA treatment, but a similar range of Förster distances as the yellow–red ones. c, FRET ratio changes (in per cent) of several variants tested with two different fluorophore pairs. Variants showing a strong response with one fluorophore pair usually also show a strong response with another pair (correlation coefficient = 0.6). d–j, Effects of mutations in linkers. d, Structure of the construct. The IAA-binding TrpR variants were cloned as tandem repeats into the construct containing donor and acceptor fluorophores, analogous to ref. 18. The positional effect of the fluorescent proteins probably stems from slight rearrangements of the overall backbone in both TrpR subunits. Predominantly, this involves helix E of the reading-head motif, which mediates the DNA interaction in the natural function of TrpR. Because helix E is towards the C-terminal end of the chain, is it thought that fluorescent proteins positioned at this end will experience a larger positional relocation and thus show a more dynamic range of the FRET signal. e–g, First-round linker mutations. All three linkers were mutated, but no pattern for the optimal linker length could be determined. One linker II variant was chosen for further mutations. h, i, Second-round linker mutations. Linkers I and III were mutated in the variant obtained in the first round, with no changes in the optimized linker II. j, Third-round linker mutations. Linker I was further mutated in the variant containing mutations in linkers II and III. The linker length axis indicates the number of amino acid residues.