Fig. 1. Crystal structure of the FIIND of rNLRP1.
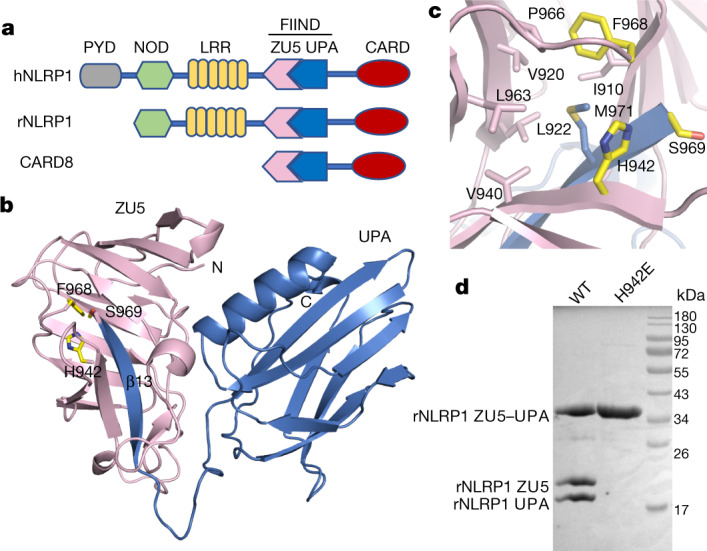
a, Schematic of domain structures of hNLRP1, rNLRP1 and CARD8. CARD, caspase activation and recruitment domain; PYD, pyrin domain. b, Crystal structure of the rNLRP1 FIIND. The ZU5 and UPA subdomains are shown in pink and blue, respectively. The catalytic residues of the FIIND are labelled and shown in stick representation. c, A close-up view of the catalytic site of the FIIND. d, Mutation of the catalytic residue H942 abolishes autoproteolysis of the rNLRP1 FIIND. Wild-type and H942 mutant rNLRP1 FIIND proteins were purified from insect cells and visualized by SDS–PAGE followed by Coomassie blue staining. See Supplementary Fig. 1 for gel raw data.
