Figure 2: Potential molecular crosstalk between autophagy and endosome biogenesis leading to Nef release from HIV infected glial cells.
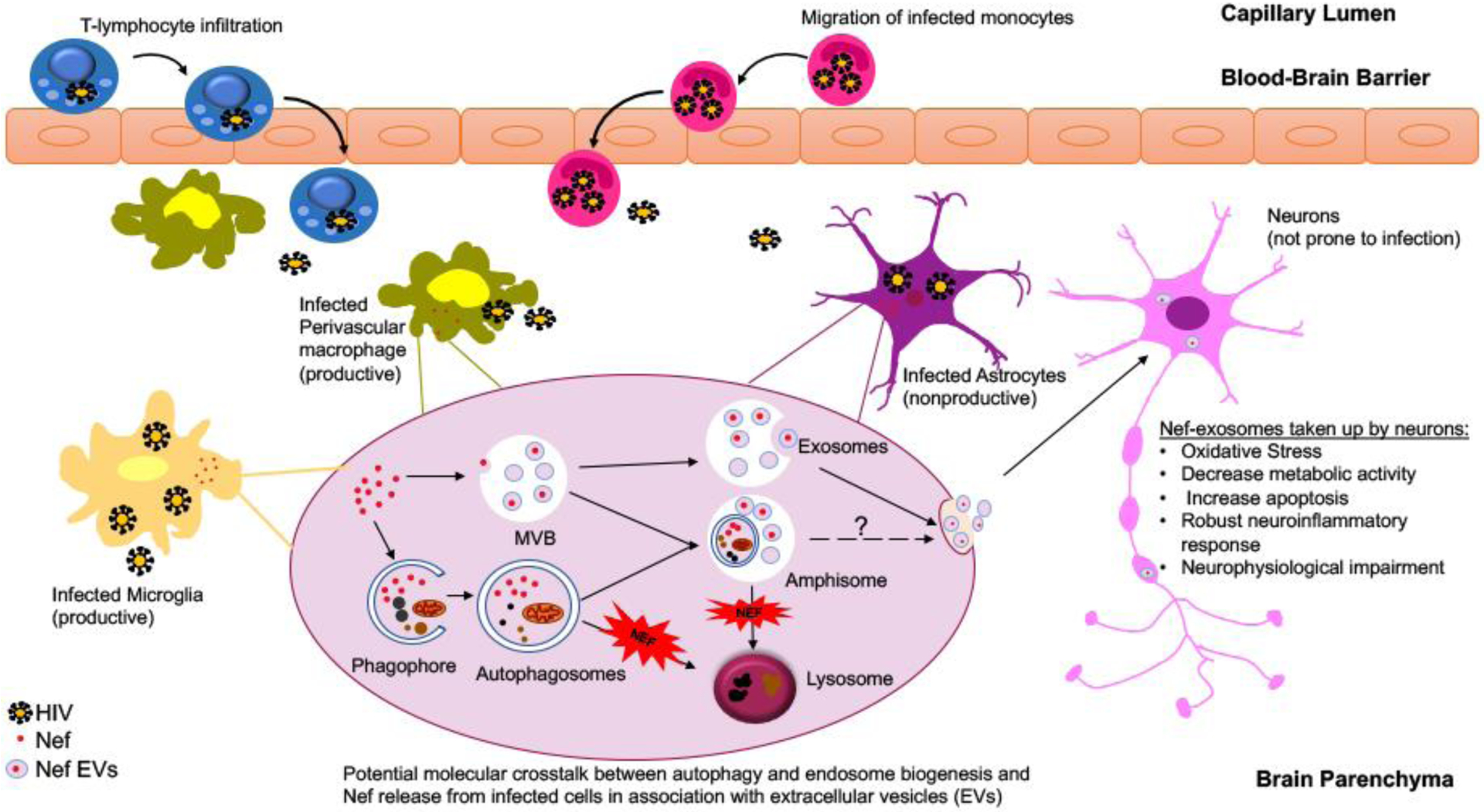
Several models were put forth to explain how cell-associated virus can be responsible for HIV infection of the brain. Infected T-lymphocytes and monocyte-derived macrophages can migrate into the brain from the peripheral circulation and seed the virus in the brain. While perivascular macrophages and microglia are considered as the viral reservoirs supporting the productive viral infection in the brain, HIV establishes infection in astrocytes by abortive infection leading to viral replication and release of viral proteins such as Nef. Limited yet accumulating data suggest a prominent role for Nef release from infected cells through two pathogenic processes, cellular autophagy and extracellular vesicle biogenesis and potentially by utilizing an establishing crosstalk between these two pathways. Within the HIV infected cells, Nef blocks autophagy by inhibiting the fusion of autophagosome with lysosomes. Autophagosomes may fuse with multivesicular bodies (MVBs) to produce organelles termed amphisomes, which can subsequently either fuse with lysosomes for content degradation or fuse the plasma membrane and secrete their content in the extracellular matrix. Released Nef exosomes can be picked up by the neurons leading to neuronal toxicity and impairment.
