Abstract
Objective:
Despite some progress in recent years, colorectal cancer (CRC) screening adherence in the United States is still suboptimal, particularly among disadvantaged groups. In this study, we assessed the association between socioeconomic status (SES) and self-reported screening non-adherence (SNA) in a sample of racial/ethnic minorities living in San Francisco, California.
Design/Methods:
A total of 376 participants of the San Francisco version of the Health Information National Trends Survey (SF-HINTS) with ages 50–75 years were included in this cross-sectional study. SNA was defined as not reporting blood stool test within the past year and not reporting sigmoidoscopy/colonoscopy within the past 10 years. Poisson regression models with robust variance estimators were used to evaluate the relation of SES with SNA, adjusting for measured confounders. Results are reported as prevalence ratios (PR) and 95% confidence intervals (95% CI).
Results:
Overall SNA was 40%. In multivariable models including all respondents, retired participants had significantly lower SNA prevalence than employed participants (PR=0.46, 95% CI=0.26 0.83). In stratified analyses by race/ethnicity, Black respondents with less than high school (PR=1.93, 95% CI=1.09, 3.43) and those with high school or equivalent (PR=1.88, 95% CI=1.16, 3.04) had significantly higher SNA prevalence than those with at least some college. Among non-Hispanic Asian/Pacific Islanders, those disabled had significantly higher prevalence of SNA as compared to employed people (PR=4.26, 95% CI=2.11, 8.60). None of the SES indicators were significantly associated to SNA among Hispanics.
Conclusions:
Participants with lower SES characteristics were less likely to adhere to CRC screening guidelines and being retired was a predictor of compliance. There was evidence of heterogeneity in associations between SES and CRC screening by race/ethnicity. Life circumstances of retired people could provide insights for designing interventions aimed to improve CRC screening uptake in these priority groups. Future efforts should consider mechanisms underlying differences by race/ethnicity.
Keywords: socioeconomic status, colorectal cancer, screening, non-adherence, racial/ethnic minorities, San Francisco
Introduction
In the United States, cancer is the second most common cause of death after cardiovascular diseases, with more than 600,000 cancer-related deaths estimated to occur this year (American Cancer Society 2020). In San Francisco, an urban setting with higher than average levels of access to care as well as high income inequality (San Francisco Department of Public Health 2019), cancer is the leading cause of death with an age-adjusted mortality rate of 129 deaths per 100,000 individuals (California Department of Public Health 2018). Among all cancer types, colorectal cancer (CRC) is the third most common type diagnosed in both women and men (California Cancer Registry 2018a), and it is the second leading cause of cancer death in the city (California Cancer Registry 2018b).
As with the rest of the United States, CRC disparities by race/ethnicity have historically been reported in San Francisco, with poorer outcomes observed in minority groups (non-Hispanic Black (Black), Hispanic and non-Hispanic Asian/Pacific Islander (API)) individuals compared to non-Hispanic White individuals (California Cancer Registry 2018c,d; Singh and Jemal 2017). Considering the great potential for early detection and more successful treatment that can result from CRC screening, screening behaviors in these groups could explain some of the differences reported in incidence and mortality (Green and Coronado 2014; Martinsen et al. 2016). Screening tests reduce the burden of CRC preventing the development of tumors, and when developed, detecting them at early stages (Meester et al. 2015). Despite some improvements during the last years, adherence to screening guidelines in the United States remains suboptimal (Hall et al. 2018; Siegel et al. 2017), especially among racial/ethnic minorities (Gray et al. 2017). Recent estimates of CRC screening non-adherence (SNA) range from 33% to 37% in the United States (American Cancer Society 2017; Centers for Disease Control and Prevention 2016) and from 30% to 32% in California (Berkowitz et al. 2018). These estimates are higher than the targets proposed by panels of experts endorsed by medical and public health agencies, such as Healthy People 2020 (29.5%) (U.S. Department of Health and Human Services and Office of Disease Prevention and Health Promotion 2012) and the National Colorectal Cancer Roundtable (20%) (American Cancer Society 2018).
Previous studies have shown that younger age, male sex, lower educational attainment, lower income, unemployment, not having a partner, not having access to care and being racial/ethnic minority are all associated with CRC SNA (Fedewa et al. 2017; Ilunga Tshiswaka et al. 2017; Shapiro et al. 2012; Shariff-Marco et al. 2013; Shih, Elting, and Levin 2008). However, information is lacking on the interplay between race/ethnicity, socioeconomic status (SES) and CRC screening behaviors in contexts in which access to care is mostly available. In 2007, the San Francisco Department of Public Health implemented Healthy San Francisco, a program designed to serve as a health care alternative for residents without health insurance but who do not qualify for Medicaid or Medicare (San Francisco Department of Public Health 2017). Also in 2011, under the program titled Low Income Health Plan, the state of California extended Medicaid eligibility to people with incomes up to 200% of the poverty line, reducing the number of uninsured individuals by 48% between 2012 and 2016 (Charles et al. 2019; Golberstein, Gonzales, and Sommers 2015). Thus, San Francisco’s diverse population and expansion of health care options for individuals of low income and the uninsured, offer a unique opportunity for evaluating CRC screening behaviors at the intersection of SES and race/ethnicity, and to understand what additional barriers for screening might exist among vulnerable groups in the presence of access to care.
In this study, we determined the prevalence of CRC SNA and assessed whether SES indicators (i.e., education, employment status and household income) were predictors of SNA in a sample of predominantly insured racial/ethnic minorities living in San Francisco. Although not all of these components of SES indicators may be modifiable by the age at which CRC screening is recommended (i.e. 50–75 years), this information could be useful for designing and targeting interventions that support the needs of these groups and aim to increase CRC screening and improve related outcomes.
Materials and Methods
Study design, study population and data collection
Data for this cross-sectional study was obtained from the San Francisco version of the Health Information National Trends Survey (HINTS) (Khoong et al. 2019). This survey was conducted as part of the research activities of the San Francisco Cancer Initiative (SF CAN), a collaborative effort between academic centers, health care providers, government, community groups, and residents to reduce the impact of cancer in the city (Hiatt et al. 2018). HINTS has been conducted all over the United States since 2003, as a National Cancer Institute initiative to understand how people are getting and using health related information as well as their risk behaviors and perceptions related to cancer. SF-HINTS builds upon this, and included additional measures intended to better characterize a diverse population often excluded from research: non-speaking English individuals, and racial and ethnic minorities.
In order to reach the target population, community-based snowball sampling was used with predefined proportions of the total sample corresponding to specific characteristics, specifically language and race/ethnicity. Half of all interviews were conducted in English (of those, 50% were with African American respondents) and the other half were conducted in Spanish (25%) and Cantonese or Mandarin (25%). Recruitment took place from May to September of 2017 at popular community locations in San Francisco where the population of interest was accessible (e.g., street markets, parks, community events). Surveys were administered face-to-face by the research team using iPads. If participants were comfortable, they were able to take the survey on their own. Data were captured using the Research Electronic Data Capture (REDCap) web-based application hosted at the University of California, San Francisco (Harris et al. 2009, 2019). This study was approved by the University of California, San Francisco Institutional Review Board.
Variables
The outcome variable in this study was CRC SNA, defined as not reporting a blood stool test (either fecal occult blood test (FOBT) or fecal immunochemical test (FIT)) within past year and not reporting a sigmoidoscopy or colonoscopy within past 10 years, according to the United States Preventive Services Task Force screening guidelines for CRC (U. S. Preventive Services Task Force et al. 2016).
The predictor variables consisted of indicators of SES: education, employment status and household income. Education was measured as the highest level of education completed and it included: less than 8 years, 8 through 11 years, 12 years completed or high school, post high school other than college, some college, college graduate and postgraduate. Based on the distribution in the sample this variable was recoded to indicate less than high school, high school and post high school. Participants were also asked about their current employment status and it was categorized as: employed, unemployed, retired, or disabled (i.e., people not working due to a disability). For household income, participants reported their past year combined annual income for all members of the family or persons living in the household. Options were predetermined and reclassified for analysis into the following categories: <$10000, $10000 to <$20000 and ≥$20000.
Additional variables were considered in the analysis to evaluate the prevalence of SNA by these characteristics or treated them as potential confounders. These included: age (continuous and 50–60, 61–75), sex at birth (male or female), marital status (single/divorced/separated/widowed or married/domestic partnership/living as married), and race/ethnicity (Black, Hispanic, or API). Health insurance coverage (Medicaid, Medicare, private, other, or none) and access to care, defined as times received care in last year (0, 1–2, 3–4 more than 4), were considered mediators and therefore, were not included in the statistical models. A depiction of the hypothesized relationship between variables considered in the study and motivation for the selection of control variables is presented in a directed acyclic graph (DAG) in Figure 1.
Figure 1.
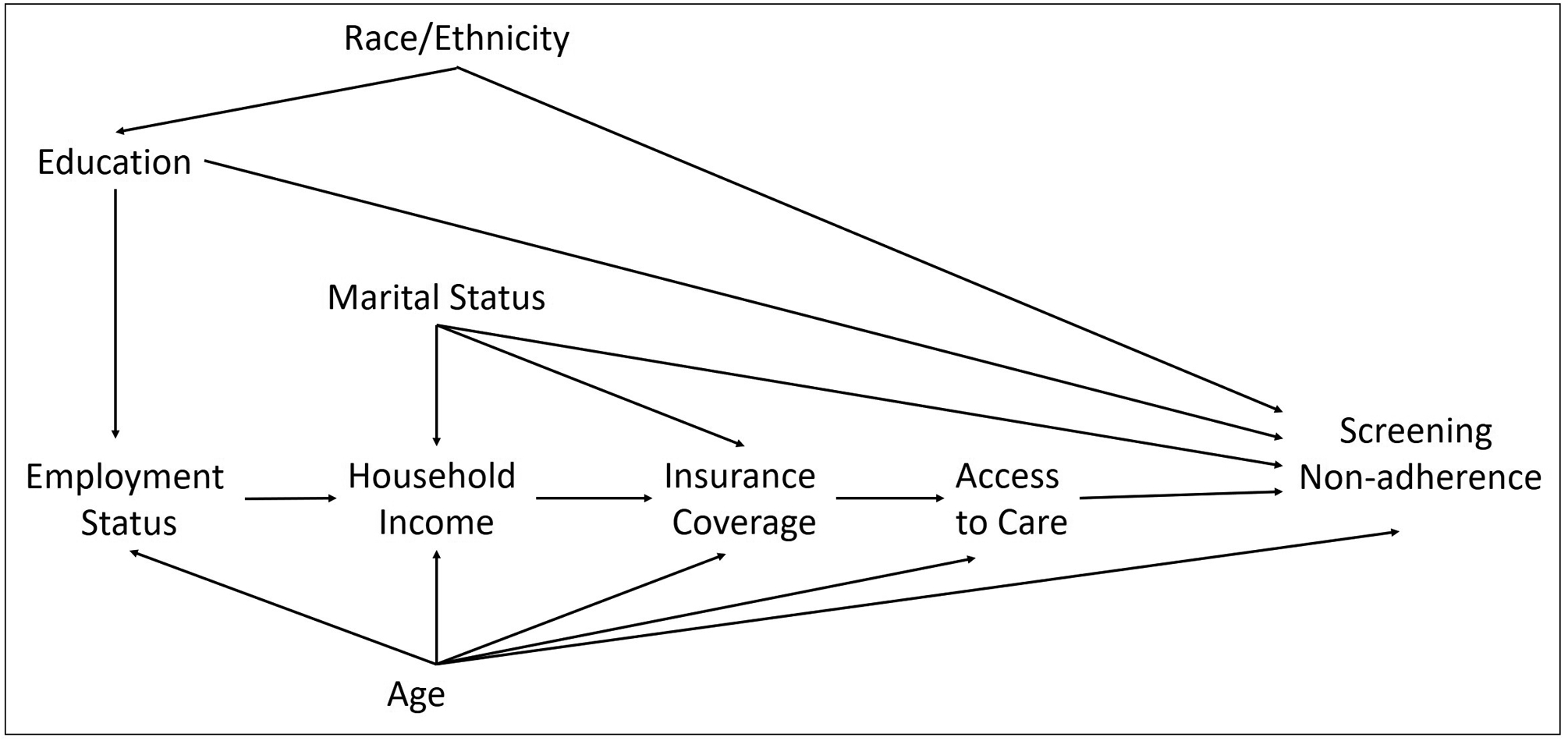
Directed acyclic graph associating SES indicators and CRC screening non-adherence.
Statistical analysis
Descriptive analyses were conducted to summarize the characteristics of the population under study and to determine the prevalence of SNA. Differences in the prevalence of SNA by participant characteristics were evaluated using Chi-squared test or Fisher’s exact test. Poisson regression models with robust variance estimators were used to assess the association of SES indicators with SNA adjusting for possible confounders. The same models stratifying by race/ethnicity were also implemented to evaluate whether SES indicators were differentially associated to SNA within subgroups. Results are reported as prevalence ratios (PR) with corresponding 95% confidence intervals (Barros and Hirakata 2003). Covariates included in the multivariable models differed for each SES indicator based on the DAG, but age and sex at birth were added to all models. Goodness-of-fit tests were conducted after running each model and fit was ascertained in all of them.
As described previously, the outcome variable was defined according to non-adherence to two different screening modalities, annual FOBT/FIT or colonoscopy/sigmoidoscopy in the last 10 years. Previous studies have reported different predictors for different CRC screening tests (de Moor et al. 2018; Shapiro et al. 2012). Therefore, additional analyses were conducted separating each type of test to evaluate the relationship between SNA and SES indicators for both modalities.
In the descriptive analyses we identified missing information on the three exposure variables (i.e., missing data for: income 11%, employment status 3%, educational level 1%). Sensitivity analyses were carried out to evaluate whether different results would have been observed with all cases having complete information. Multiple imputation techniques using chained equations were implemented (White, Royston, and Wood 2011). A total of ten multiply imputed datasets were created and estimates were combined following Rubin’s rules (Rubin 1987).
Finally, to evaluate the potential role of Medicare on SNA among older individuals, we repeated the main analysis and restricted it to participants who reported having this type of coverage (n=135). All analyses were conducted in Stata (Version 15.1, College Station, TX, USA).
Results
From the 1,027 participants enrolled in the study, 651 were excluded because: their age was out of the range in which screening guidelines are applicable (50–75 years, n=566), had prior history of cancer (n=57) and reported being white or other race (n=28); for a total of 376 individuals included in the analysis. The sample was comprised of 37% API, 32% Hispanic, and 31% Black respondents. Median (IQR) age was 60 (55–66) and 60% were female. One third of participants had an education of less than high school, 26% were unemployed, 29% reported an annual household income of less than $10,000 and 89% indicated having some type of health insurance coverage. Overall SNA was 40%. Prevalence of SNA was higher in younger people, males, those without a partner, Black respondents, and those who were less educated, disabled, unemployed, and who reported the lowest annual income category. A detailed description of participant characteristics and prevalence of SNA by these variables can be found in Table 1.
Table 1.
Characteristics of study population and CRC screening non-adherence (N=376)
| n (%) | % CRC SNA | p-value | |
|---|---|---|---|
| Age, median (IQR) | 60 (55–66) | --- | --- |
| Age | |||
| 50–60 | 194 (51.6) | 49.0 | <0.001 |
| 61–75 | 182 (48.4) | 30.8 | |
| Sex at birth | |||
| Female | 224 (59.6) | 39.3 | 0.68 |
| Male | 152 (40.4) | 41.5 | |
| Marital status | |||
| Single/divorced/separated/widowed | 231 (61.4) | 42.0 | 0.38 |
| Married/domestic partnership/living as married | 139 (37.0) | 37.4 | |
| Race/ethnicity | |||
| Non-Hispanic Black | 117 (31.1) | 47.9 | 0.11 |
| Hispanic | 120 (31.9) | 38.3 | |
| Non-Hispanic Asian/Pacific-Islander | 139 (37.0) | 35.3 | |
| Education | |||
| Less than high school | 122 (32.4) | 38.5 | 0.36 |
| High school | 134 (35.6) | 44.0 | |
| Post high school | 116 (30.9) | 35.3 | |
| Employment status | |||
| Unemployed | 98 (26.1) | 52.0 | <0.001 |
| Disabled | 71 (18.9) | 52.1 | |
| Retired | 85 (22.6) | 16.5 | |
| Employed | 111 (29.5) | 39.6 | |
| Household income | |||
| <$10,000 | 107 (28.5) | 43.9 | 0.46 |
| $10,000 to <$20,000 | 131 (34.8) | 36.6 | |
| ≥$20,000 | 97 (25.8) | 37.1 | |
| Health insurance coverage | |||
| None | 42 (11.2) | 59.5 | 0.02 |
| Medicaid | 72 (19.2) | 27.8 | |
| Medicare | 138 (36.7) | 39.1 | |
| Private | 66 (17.6) | 36.4 | |
| Other | 42 (11.2) | 35.7 | |
| Times received care in last 12 months (no ER) | |||
| 0 | 57 (15.2) | 64.9 | <0.001 |
| 1–2 | 115 (30.6) | 40.9 | |
| 3–4 | 106 (28.2) | 32.1 | |
| ≥ 5 | 98 (26.1) | 33.7 | |
| SNA (no FOBT/FIT within past year and no sigmoidoscopy/colonoscopy within past 10 years) | 151 (40.2) | --- | --- |
Results of the multivariable models for CRC SNA are shown in Table 2. Participants with lower educational attainment had higher prevalence of SNA, but results did not reach statistical significance (less than high school: PR= 1.33, 95% CI=0.95, 1.86; high school: PR= 1.30, 95% CI=0.95, 1.77; compared to post high school). Compared to employed participants, those unemployed (PR=1.24, 95% CI=0.90, 1.69) and disabled (PR=1.31, 95% CI=0.95, 1.81) had higher but not statistically significant SNA prevalence, and retired people had significantly lower SNA prevalence (PR=0.46, 95% CI=0.26, 0.83). We found no differences in SNA by household income levels. After conducting multiple imputation and repeating all analyses, similar results were obtained (Table 2). Effect estimates for all covariates are available in the Supplemental Table.
Table 2.
Relationship between SES indicators and CRC screening non-adherence
| Complete Case Analysis | Analysis After Multiple Imputation | |||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted PR (95% CI) | p-value | Adjusted* PR (95% CI) | p-value | Unadjusted PR (95% CI) | p-value | Adjusted* PR (95% CI) | p-value | |
| Education^ | ||||||||
| Less than HS | 1.09 (0.78, 1.52) | 0.61 | 1.33 (0.95, 1.86) | 0.10 | 1.08 (0.77, 1.51) | 0.65 | 1.31 (0.94, 1.83) | 0.11 |
| HS or equivalent | 1.25 (0.91, 1.70) | 0.17 | 1.30 (0.95, 1.77) | 0.10 | 1.23 (0.90, 1.68) | 0.18 | 1.28 (0.94, 1.75) | 0.11 |
| At least some college | Ref | Ref | Ref | Ref | ||||
| Employment status^^ | ||||||||
| Unemployed | 1.31 (0.97, 1.77) | 0.07 | 1.23 (0.90, 1.69) | 0.19 | 1.32 (0.99, 1.78) | 0.06 | 1.27 (0.94, 1.72) | 0.12 |
| Disabled | 1.31 (0.95, 1.81) | 0.09 | 1.31 (0.95, 1.80) | 0.10 | 1.31 (0.95, 1.80) | 0.10 | 1.30 (0.95, 1.79) | 0.10 |
| Retired | 0.42 (0.24, 0.71) | 0.001 | 0.46 (0.26, 0.83) | 0.009 | 0.42 (0.25, 0.71) | 0.001 | 0.48 (0.27, 0.85) | 0.011 |
| Employed | Ref | Ref | Ref | Ref | ||||
| Household income^^^ | ||||||||
| <$10,000 | 1.18 (0.85, 1.66) | 0.33 | 1.10 (0.76, 1.60) | 0.61 | 1.18 (0.85, 1.62) | 0.32 | 1.13 (0.77, 1.64) | 0.53 |
| $10,000 to <$20,000 | 0.99 (0.70, 1.39) | 0.94 | 0.97 (0.66, 1.42) | 0.99 (0.71, 1.37) | 0.93 | 1.04 (0.73, 1.48) | 0.84 | |
| ≥$20,000 | Ref | Ref | Ref | Ref | ||||
Adjusted for:
sex at birth, age, race/ethnicity;
sex at birth, age, education;
sex at birth, age, education, employment status, marital status
In stratified analyses, educational attainment was a predictor of SNA among Black respondents, and employment status was a predictor of SNA among API respondents (Table 3). Compared to having at least some college, Black respondents with less than high school (PR=1.93, 95% CI=1.09, 3.43) and high school or equivalent (PR=1.88, 95% CI=1.16, 3.04) had a significantly higher SNA prevalence. Among API respondents, those disabled had significantly higher prevalence of SNA as compared to employed people (PR=4.26, 95% CI=2.11, 8.60). Among Hispanic respondents, those reporting lower income had higher prevalence of SNA, but results were not statistically significant (<$10,000: PR= 1.67, 95% CI=0.76, 3.66; $10,000 to <$20,000: PR= 2.12, 95% CI=1.00, 4.54; both compared to ≥$20,000).
Table 3.
Relationship between SES indicators and CRC screening non-adherence by race/ethnicity
| Non-Hispanic Black | Hispanic | Non-Hispanic Asian/Pacific Islander | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted PR (95% CI) | p-value | Adjusted* PR (95% CI) | p-value | Unadjusted PR (95% CI) | p-value | Adjusted* PR (95% CI) | p-value | Unadjusted PR (95% CI) | p-value | Adjusted* PR (95% CI) | p-value | |
| Education^ | ||||||||||||
| Less than HS | 2.04 (1.15, 3.61) | 0.014 | 1.93 (1.09, 3.43) | 0.025 | 1.03 (0.60, 1.77) | 0.92 | 1.10 (0.64, 1.92) | 0.72 | 0.81 (0.45, 1.45) | 0.48 | 1.15 (0.64, 2.09) | 0.64 |
| HS or equivalent | 1.92 (1.19, 3.11) | 0.008 | 1.88 (1.16, 3.04) | 0.010 | 0.91 (0.50, 1.65) | 0.77 | 0.96 (0.54, 1.72) | 0.90 | 0.89 (0.48, 1.63) | 0.70 | 1.09 (0.61, 1.95) | 0.78 |
| At least some college | Ref | Ref | Ref | Ref | Ref | Ref | ||||||
| Employment status^^ | ||||||||||||
| Unemployed | 2.13 (1.11, 4.11) | 0.024 | 1.56 (0.79, 3.07) | 0.20 | 0.91 (0.54, 1.52) | 0.71 | 0.94 (0.56, 1.59) | 0.82 | 1.35 (0.83, 2.19) | 0.23 | 1.55 (0.91, 2.63) | 0.11 |
| Disabled | 2.21 (1.16, 4.20) | 0.015 | 1.84 (0.97, 3.48) | 0.06 | 0.62 (0.30, 1.25) | 0.18 | 0.62 (0.30, 1.26) | 0.19 | 2.38 (1.63, 3.45) | <0.001 | 4.26 (2.11, 8.60) | <0.001 |
| Retired | 0.60 (0.15, 2.45) | 0.48 | 0.55 (0.14, 2.12) | 0.38 | 0.47 (0.16, 1.36) | 0.16 | 0.55 (0.17, 1.77) | 0.32 | 0.36 (0.18, 0.74) | 0.005 | 0.72 (0.29, 1.78) | 0.48 |
| Employed | Ref | Ref | Ref | Ref | Ref | Ref | ||||||
| Household income^^^ | ||||||||||||
| <$10,000 | 2.64 (1.31, 5.30) | 0.007 | 1.62 (0.76, 3.45) | 0.21 | 1.40 (0.71, 2.76) | 0.34 | 1.67 (0.76, 3.66) | 0.20 | 0.55 (0.30, 0.99) | 0.045 | 0.72 (0.38, 1.37) | 0.32 |
| $10,000 to <$20,000 | 2.13 (1.03, 4.40) | 0.04 | 1.35 (0.62, 2.94) | 0.45 | 1.49 (0.74, 3.00) | 0.27 | 2.12 (1.00, 4.54) | 0.05 | 0.46 (0.28, 0.77) | 0.003 | 0.53 (0.25, 1.12) | 0.10 |
| ≥$20,000 | Ref | Ref | Ref | Ref | Ref | Ref | ||||||
Adjusted for:
sex at birth, age;
sex at birth, age, education;
sex at birth, age, education, employment status, marital status
When evaluating the relationship between SES and SNA to the annual FOBT/FIT, we found similar results for all categories of educational attainment and household income. However, compared to employed participants, those disabled (PR=1.28, 95% CI=1.04, 1.58) had significantly higher SNA prevalence. Unemployed people had higher SNA prevalence (PR=1.18, 95% CI=0.96, 1.44) and retired people had lower (PR=0.81, 95% CI=0.59, 1.11), but these results were not statistically significant (Table 4).
Table 4.
Relationship between SES indicators and CRC screening non-adherence by test modality
| FOBT/FIT | Sigmoidoscopy/Colonoscopy | |||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted PR (95% CI) | p-value | Adjusted* PR (95% CI) | p-value | Unadjusted PR (95% CI) | p-value | Adjusted* PR (95% CI) | p-value | |
| Education^ | ||||||||
| Less than HS | 0.92 (0.75, 1.14) | 0.46 | 0.99 (0.81, 1.22) | 0.95 | 1.09 (0.89, 1.32) | 0.41 | 1.22 (1.01, 1.48) | 0.04 |
| HS or equivalent | 1.04 (0.87, 1.26) | 0.65 | 1.07 (0.89, 1.29) | 0.45 | 1.10 (0.91, 1.33) | 0.32 | 1.12 (0.93, 1.36) | 0.22 |
| At least some college | Ref | Ref | Ref | Ref | ||||
| Employment status^^ | ||||||||
| Unemployed | 1.17 (0.96, 1.42) | 0.13 | 1.18 (0.96, 1.44) | 0.12 | 1.13 (0.95, 1.35) | 0.17 | 1.10 (0.91, 1.31) | 0.32 |
| Disabled | 1.26 (1.03, 1.54) | 0.023 | 1.28 (1.04, 1.58) | 0.019 | 1.03 (0.83, 1.27) | 0.80 | 1.02 (0.83, 1.26) | 0.82 |
| Retired | 0.76 (0.58, 1.00) | 0.05 | 0.81 (0.59, 1.11) | 0.19 | 0.72 (0.55, 0.93) | 0.013 | 0.77 (0.57, 1.05) | 0.09 |
| Employed | Ref | Ref | Ref | Ref | ||||
| Household income^^^ | ||||||||
| <$10,000 | 1.00 (0.81, 1.23) | 0.97 | 0.93 (0.74, 1.18) | 0.56 | 1.21 (0.98, 1.49) | 0.07 | 1.19 (0.94, 1.51) | 0.15 |
| $10,000 to <$20,000 | 0.93 (0.76, 1.15) | 0.53 | 0.88 (0.68, 1.12) | 0.29 | 1.08 (0.87, 1.33) | 0.49 | 1.13 (0.90, 1.42) | 0.30 |
| ≥$20,000 | Ref | Ref | Ref | Ref | ||||
Adjusted for:
sex at birth, age, race/ethnicity;
sex at birth, age, education;
sex at birth, age, education, employment status, marital status
For sigmoidoscopy/colonoscopy, we observed a trend of higher SNA prevalence in people with lower educational levels (less than high school: PR=1.22, 95% CI=1.01, 1.48; high school: PR= 1.12, 95% CI=0.93, 1.36; all compared to post high school). Those unemployed (PR=1.10, 95% CI=0.91, 1.31) and disabled (PR=1.02, 95% CI=0.83, 1.26) had similar SNA prevalence as compared to employed participants. A lower SNA prevalence was observed for retired people (PR=0.77, 95% CI=0.57, 1.05) but results were not statistically significant. Higher prevalence of SNA occurred in participants reporting lower earnings (<$10,000: PR= 1.19, 95% CI=0.94, 1.51; $10,000 to <$20,000: PR= 1.13, 95% CI=0.90, 1.42; all compared to ≥$20,000) but results did not achieve statistical significance (Table 4).
In the sensitivity analysis among people reporting having Medicare, we found that compared to employed participants those retired had significantly lower SNA prevalence (PR=0.32, 95% CI=0.14, 0.74), and no differences were observed for unemployed (PR=0.84, 95% CI=0.43, 1.67) and disabled people (PR=1.17, 95% CI=0.68, 2.02). For education and household income, we found no differences in SNA.
Discussion
In this study, we analyzed data from a multiethnic and socioeconomically disadvantaged population in San Francisco, an urban city that has made substantial efforts to ensure access to care for all of its residents. We found a high overall prevalence of reported CRC SNA, and that employment status was a predictor of SNA in the whole study population; in particular, retirement predicted a substantially lower prevalence of SNA. We also evaluated the association of SNA with education and household income, and in adjusted analyses found no differences in SNA prevalence by levels of these two factors. In stratified analyses by race/ethnicity, lower educational attainment and being disabled, were factors associated to SNA in Black and API participants, respectively.
The prevalence of CRC SNA in this study was similar to other studies evaluating racial/ethnic groups in urban settings. For example, a study assessing screening behaviors in a racial/ethnically diverse population residing in Houston, Texas, reported CRC SNA among minorities was 44% (Calo et al. 2015). Another study conducted in East Harlem, New York found the prevalence of CRC SNA among Hispanics was 42% (Ellison et al. 2011). Also, for Chinese Americans residing in California the prevalence of CRC SNA was 49% (Sentell et al. 2015). Interestingly, when compared to national estimates based on the results of the National Health Interview Survey (NHIS) for 2015 (Sauer et al. 2019), SNA in our study was lower for Hispanic (38% vs 50%) and API (35% vs 51%) respondents, but higher for Black respondents (48% vs 38%). A possible explanation for the lower CRC SNA in some groups of our study is that despite their low SES characteristics, a high proportion of participants reported having health insurance coverage and access to care during the last year, factors linked to being up to date with CRC screening (de Moor et al. 2018).
The higher than average access to care in San Francisco allowed us to evaluate the role of SES indicators on screening behaviors when healthcare opportunities are available for under-represented groups. The finding that retired people were more likely to adhere to CRC screening in the whole study group (and although not statistically significant, with similar effect estimates within racial/ethnic groups), aligns with results from previous studies. Using data from the 2014 Health Center Patient Survey, Lin et al. (2017) reported participants not in the labor force (retired) were twice as likely to be compliant with CRC screening than those employed. Similarly, analyzing data from NHIS for the years 2010, 2013 and 2015, Fedewa et al. (2017) evaluated cancer screening behaviors by occupational characteristics and found that compared to employed people, prevalence of CRC screening was higher in retired participants and lower in those unemployed. Also, Rastogi et al. (2019) evaluated disparities in CRC screening in the New York City Community Health Survey and reported that those not in labor force were more likely to be up to date with CRC screening recommendations than unemployed participants.
Several explanations have been proposed to understand the finding of lower CRC SNA in retired people, including: having more time available to attend medical appointments, being financially stable, having access to healthcare through Medicare, and an increased motivation to preserve health at later stages of life through health care seeking activities such as cancer screening (Rastogi et al. 2019). In this study, however, we do not believe Medicare coverage is the explanation for retired people reporting lower CRC SNA. In multivariable models, insurance status and access to care were not included as covariates because we consider they were not confounders of the relationship of interest but mediators (Figure 1). In an assessment of Medicare as the potential driver of the observed findings, retired participants still had lower SNA prevalence than those employed, and no differences were observed for unemployed and disabled people. This suggests that Medicare might not be the only mechanism for retired participants’ adherence to CRC screening guidelines in this sample.
Being employed could have benefits that promote health seeking behaviors (i.e., following CRC screening recommendations), such as health insurance coverage and paid sick leave, but these benefits are not inherent to all jobs. In this study we did not collect information about jobs (e.g., part-time vs. full-time, number of jobs, employer, number of employees in the company or organization, occupation). However, we looked at the distribution of total annual household income in those who reported being employed. We found that 39% reported having a household income of less than $20,000 and 72% reported a household income of less than $50,000. Even assuming an unlikely scenario in which the household income reflected the full salary of employed participants, this suggests that a high proportion of employed respondents were earning low levels of income, with potentially limited or no health benefits. As this study took place in California, where Medicaid was expanded to cover low-income individuals (Golberstein, Gonzales, and Sommers 2015), lacking this job-related benefit is not an issue. However, other factors such as not having paid sick leave or compensatory time to attend medical appointments, along with the possibility of job insecurity in a city with high cost of living could have been present in this population, accounting for the higher CRC SNA prevalence in the employed group (Lin et al. 2017; Peipins et al. 2012).
Increasing CRC screening in minority groups has been proposed as a strategy to reduce health disparities, but to accomplish this, it is important to consider features of available tests, and characteristics and preferences of individuals (Gray et al. 2017; Green and Coronado 2014; Martinsen et al. 2016; Wilcox et al. 2015). Our findings in the stratified analyses, despite sample size limitations, support the importance of contemplating groups’ characteristics and suggest that SES indicators might contribute differently to screening behaviors by race and ethnicity. In this study, we also examined the association between SES and the different screening modalities, and predictors for individuals not following recommendations for each test were identified. For FOBT/FIT, we found disabled participants were less likely to complete the annual test than employed participants. This result is consistent with studies that suggest that stool-based screening requires efforts to collect and handle the samples which might be difficult for people with disabilities (Gray et al. 2017; Sentell et al. 2015). For sigmoidoscopy/colonoscopy, participants with lower educational level were more likely to not follow screening recommendations. Completion of these tests involve understanding them (i.e., benefits and potential risks) and following instructions and procedures. Therefore, the level of education attained and literacy skills could influence whether people are capable or not of preparing for the tests and receiving them (Gray et al. 2017; Sentell et al. 2015; Shneyderman et al. 2016).
In order to increase CRC screening in this diverse and disadvantaged population but with high levels of insurance coverage, health systems-level interventions that have shown to be successful in similar settings should be evaluated and implemented. Patient navigation, defined as “services that improve engagement in healthcare by providing personal guidance through the healthcare system” (Nelson et al., “Patient Navigation and Cancer Screening”, 2020), is one of such strategies with extensive evidence of favorable results. For example, in a randomized controlled trial (RCT) conducted among ethnically diverse and low-income communities in Massachusetts, Lasser et al. (2011) reported that compared to usual care, patient navigation increased the rates of CRC screening. They also found that the intervention was especially beneficial for Black individuals and non-English speakers, groups in which screening rates doubled as compared to usual care (Lasser et al. 2011). Also, in a recent meta-analysis evaluating the effectiveness of patient navigation for cancer screening in populations adversely affected by health disparities, researchers found that in 23 out of 28 studies targeting CRC screening, groups assisted by patient navigators had higher screening rates (Nelson et al., “Patient Navigation and Cancer Screening”, 2020). When combining results of these studies, patient navigation was associated with higher CRC screening rates overall, and across different study characteristics: study design (i.e., RCTs and observational studies), screening modalities (i.e., FOBT/FIT and colonoscopy), screening behaviors (i.e., individuals adherent and non-adherent to screening recommendations), and follow-up time (i.e., 6 months, 1 year, 18 months and 5 years) (Nelson et al., “Patient Navigation and Cancer Screening”, 2020). Although patient navigation would help to address personal, logistical and system barriers for CRC screening (Krok-Schoen, Oliveri and Paskett 2016), the implementation of other strategies, including telephone calls and prompts should be explored in this population, as they have also shown to increase cancer screening rates in similar settings (Nelson et al., “Achieving Health Equity in Preventive Services”, 2020).
This study has several limitations. First, although the snowball sampling methodology was effective at enrolling a multi-ethnic cohort of low-income respondents, this approach resulted in a non-probability sample. Results may therefore not generalize to San Francisco in particular or United States urban settings in general because the study sample has a different distribution of important characteristics (e.g., SES), and is not representative of these other populations. In addition, there may be sample selection bias present if respondents who participated in the study differed substantially from the target population of multi-ethnic, low-income residents of San Francisco by SES and/or health-seeking behaviors, including screening. Second, all variables evaluated in the study were collected using a questionnaire and self-report. Hence, there is potential for misclassification of the outcome as people could tend to report higher adherence to screening than reality (i.e. social desirability bias); this could result in underestimation of SNA and biased estimates if social desirability bias varied by respondents’ SES (Rauscher et al. 2008). There is also potential for exposure misclassification, especially for variables that change over time (i.e., employment status and income) and the information collected during the interview will not necessarily correspond to the actual values when the reported outcome occurred. For example, a person may have been employed at the time of their colonoscopy five years prior but retired at the time of the interview. Based on this issue of misclassification of exposure for employment status, there is also potential for residual confounding, as employment status is a confounder of the relationship between income and SNA. Fourth, the small sample size that resulted from necessarily restricting the analysis to age eligible participants reduced our statistical power, especially in the stratified analyses. Fifth, some variables had data missing, among them the exposures of interest. Other variables with missing information were marital status (2%) and health insurance coverage (4%). This potential problem was addressed using multiple imputation and the results obtained after running the analysis again were similar to the complete case scenario.
In summary, in a multi-ethnic community-based sample in an urban setting in the United States with unique health access programs that would theoretically improve CRC screening outcomes, we found that participants with lower SES indicators had higher SNA prevalence. We observed this association even in a sample with low average SES and in a setting with higher than average access to care, given both state and local-level programs to increase access for low-income individuals. We additionally found that being retired was significantly associated with lower SNA prevalence, which coincided with findings from prior research.
Life circumstances of retired people, not only health insurance coverage nor access to care, but having time available to attend medical appointments and absence of job insecurity might be facilitators of screening adherence in this population. Also, although characteristics of individuals and features of screening tests require further definition, our findings could suggest approaches for developing interventions aimed to improve CRC screening uptake in this priority group. For example, irrespective of having health insurance, disabled people might require assistance for collecting stool samples and completing the FIT. Similarly, people with low educational attainment and access to care, might need detailed and accessible explanations to prepare for the sigmoidoscopy/colonoscopy. Lastly, the feasibility of incorporating health systems interventions that have successfully increased CRC screening rates among disadvantaged groups in other settings, such as patient navigation, telephone calls and prompts (Nelson et al., “Achieving Health Equity in Preventive Services”, 2020), should be evaluated as strategies to be implemented in this vulnerable population.
Supplementary Material
Acknowledgements
The authors would like to thank all the individuals and organizations that have participated in the design and implementation of the SF-HINTS, including Trung Nguyen, Rena Pasick, Anna María Nápoles, Mekhala Hoskote, Gem M. Le, Pamela Williams; Cynthia Cheung; Corina Liew; Alejo Perez-Stable Husni, Francisco Quintanilla. Authors also thank Maria Glymour, Mark Segal and Michelle A. DeVost for helpful comments on the first versions of the manuscript.
Funding
This work was supported by the National Institute on Aging under Grants T32AG049663 and K01AG056602; National Cancer Institute Population Cancer Center Support Grant P30CA082103; and NIH Midcareer Investigator Award in Patient-Oriented Research under Grant K24CA212294.
Footnotes
Conflicts of interest
The authors declare no potential conflicts of interest.
References
- American Cancer Society. 2017. Colorectal Cancer Facts & Figures 2017–2019. Atlanta, GA: American Cancer Society. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/colorectal-cancer-facts-and-figures/colorectal-cancer-facts-and-figures-2017-2019.pdf [Google Scholar]
- American Cancer Society. 2018. “The National Colorectal Cancer Roundtable.” Atlanta, GA: American Cancer Society. https://www.cancer.org/health-care-professionals/colon-md.html#NCCRT. [Google Scholar]
- American Cancer Society. 2020. Cancer Facts & Figures 2020. Atlanta, GA: American Cancer Society. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2020/cancer-facts-and-figures-2020.pdf [Google Scholar]
- Barros Aluísio J. D., and Hirakata VN. 2003. “Alternatives for Logistic Regression in Cross-sectional Studies: An Empirical Comparison of Models that Directly Estimate the Prevalence Ratio.” BMC Medical Research Methodology 3 (1):21. doi: 10.1186/1471-2288-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz Z, Zhang X, Richards TB, Nadel M, Peipins LA, and Holt J. 2018. “Multilevel Small-Area Estimation of Colorectal Cancer Screening in the United States.” Cancer Epidemiology Biomarkers & Prevention 27 (3):245–53. doi: 10.1158/1055-9965.EPI-17-0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- California Cancer Registry. 2018a. “Age-adjusted Invasive Cancer Incidence Rates by County in California, 2011–2015.” http://cancer-rates.info/ca/
- California Cancer Registry. 2018b. “Age-adjusted Cancer Mortality Rates by County in California, 2011–2015.” http://cancer-rates.info/ca/
- California Cancer Registry. 2018c. “Age-adjusted Invasive Cancer Incidence Rates by County in California, 1988–2015.” http://cancer-rates.info/ca/
- California Cancer Registry. 2018d. “Age-adjusted Cancer Mortality Rates by County in California, 1988–2015.” http://cancer-rates.info/ca/
- California Department of Public Health. 2018. “San Francisco County’s Health Status Profile for 2018.” Sacramento, CA: California Department of Public Health. https://www.cdph.ca.gov/Programs/CHSI/CDPH%20Document%20Library/CHSP-SANFRANCISCO.pdf [Google Scholar]
- Calo WA, Vernon SW, Lairson DR, and Linder SH. 2015. “Associations Between Contextual Factors and Colorectal Cancer Screening in a Racially and Ethnically Diverse Population in Texas.” Cancer Epidemiology 39 (6):798–804. doi: 10.1016/j.canep.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. 2016. “Quick Facts: Colorectal Cancer Screening in U.S. Behavioral Risk Factor Surveillance System-2016.” Atlanta, GA: Centers for Disease Control and Prevention. https://www.cdc.gov/cancer/colorectal/pdf/QuickFacts-BRFSS-2016-CRC-Screening-508.pdf [Google Scholar]
- Charles SA, Becker T, Perry IE, Jacobs K, Pourat N, Ditter M, Mekhaiel M, et al. 2019. “The State of Health Insurance in California: Findings from the 2015–2016 California Health Interview Survey.” Los Angeles, CA: UCLA Center for Health Policy Research. http://healthpolicy.ucla.edu/publications/Documents/PDF/2019/shicreport-nov2019.pdf [Google Scholar]
- de Moor JS, Cohen RA, Shapiro JA, Nadel MR, Sabatino SA, Yabroff KR, Fedewa S, et al. 2018. “Colorectal Cancer Screening in the United States: Trends from 2008 to 2015 and Variation by Health Insurance Coverage.” Preventive Medicine 112 (November 2017):199–206. doi: 10.1016/j.ypmed.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison J, Jandorf L, and Katherine D. 2011. “Colonoscopy Screening Information Preferences Among Urban Hispanics.” Journal of Immigrant and Minority Health. doi: 10.1017/S0950268814002131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedewa SA, Sauer AG, DeSantis C, Siegel RL, and Jemal A. 2017. “Disparities in Cancer Screening by Occupational Characteristics.” Preventive Medicine 105:311–318. doi: 10.1016/j.ypmed.2017.10.012. [DOI] [PubMed] [Google Scholar]
- Golberstein E, Gonzales G, and Sommers BD. 2015. “California’s Early ACA Expansion Increased Coverage and Reduced Out-Of-Pocket Spending for the State’s Low-Income Population.” Health Affairs (Millwood) 34 (10):1688–1694. doi: 10.1377/hlthaff.2015.0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray TF, Cudjoe J, Murphy J, Thorpe RJ Jr., Wenzel J, and Han HR. 2017. “Disparities in Cancer Screening Practices among Minority and Underrepresented Populations.” Seminars in Oncology Nursing 33 (2):184–198. doi: 10.1016/j.soncn.2017.02.008. [DOI] [PubMed] [Google Scholar]
- Green BB, and Coronado GD. 2014. ““BeneFITs” to Increase Colorectal Cancer Screening in Priority Populations.” JAMA Internal Medicine 174 (8):1242–1243. doi: 10.1001/jamainternmed.2014.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall IJ, Tangka FKL, Sabatino SA, Thompson TD, Graubard BI, and Breen N. 2018. “Patterns and Trends in Cancer Screening in the United States.” Preventing Chronic Disease 15:E97. doi: 10.5888/pcd15.170465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, McLeod L, et al. 2019. “The REDCap Consortium: Building an International Community of Software Platform Partners.” Journal of Biomedical Informatics 95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, and Conde JG. 2009. “Research Electronic Data Capture (REDCap)-A Metadata-driven Methodology and Workflow Process for Providing Translational Research Informatics Support.” Journal of Biomedical Informatics 42 (2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiatt RA, Sibley A, Fejerman L, Glantz S, Nguyen T, Pasick R, Palmer N, et al. 2018. “The San Francisco Cancer Initiative: A Community Effort to Reduce the Population Burden of Cancer.” Health Affairs (Millwood) 37 (1):54–61. doi: 10.1377/hlthaff.2017.1260. [DOI] [PubMed] [Google Scholar]
- Ilunga Tshiswaka D, Donley T, Okafor A, Memiah P, and Mbizo J. 2017. “Prostate and Colorectal Cancer Screening Uptake among US and Foreign-Born Males: Evidence from the 2015 NHIS Survey.” Journal Community Health 42 (3):612–623. doi: 10.1007/s10900-016-0296-1. [DOI] [PubMed] [Google Scholar]
- Khoong EC, Le GM, Hoskote M, Rivadeneira NA, Hiatt RA, and Sarkar U. 2019. “Health Information-seeking Behaviors and Preferences of a Diverse, Multilingual Urban Cohort.” Medical Care 57 Suppl 6 Suppl 2:S176–S183. doi: 10.1097/MLR.0000000000001050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krok-Schoen Jessica L., Oliveri Jill M., and Paskett Electra D.. 2016. “Cancer Care Delivery and Women’s Health: The Role of Patient Navigation.” Frontiers in Oncology 6 (JAN): 1–10. doi: 10.3389/fonc.2016.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasser Karen E., Murillo Jennifer, Lisboa Sandra, Casimir A. Naomie, Shah Lisa Valley, Emmons Karen M., Fletcher Robert H., and Ayanian John Z.. 2011. “Colorectal Cancer Screening among Ethnically Diverse, Low-Income Patients: A Randomized Controlled Trial. “ Archives of Internal Medicine 171 (10): 906–912. doi: 10.1001/archinternmed.2011.201. [DOI] [PubMed] [Google Scholar]
- Lin SC, McKinley D, Sripipatana A, and Makaroff L. 2017. “Colorectal Cancer Screening at US Community Health Centers: Examination of Sociodemographic Disparities and Association with Patient-provider Communication.” Cancer 123 (21):4185–4192. doi: 10.1002/cncr.30855. [DOI] [PubMed] [Google Scholar]
- Martinsen RP, Morris CR, Pinheiro PS, Parikh-Patel A, and Kizer KW. 2016. “Colorectal Cancer Trends in California and the Need for Greater Screening of Hispanic Men.” American Journal of Preventive Medicine 51 (6):e155–e163. doi: 10.1016/j.amepre.2016.05.019. [DOI] [PubMed] [Google Scholar]
- Meester RG, Doubeni CA, Lansdorp-Vogelaar I, Goede SL, Levin TR, Quinn VP, Ballegooijen Mv, Corley DA, and Zauber AG. 2015. “Colorectal Cancer Deaths Attributable to Nonuse of Screening in the United States.” Annals of Epidemiology 25 (3):208–213 e1. doi: 10.1016/j.annepidem.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson Heidi D., Cantor Amy, Wagner Jesse, Jungbauer Rebecca, Ana Quiñones Lucy Stillman, and Kondo Karli. 2020. “Achieving Health Equity in Preventive Services: A Systematic Review for a National Institutes of Health Pathways to Prevention Workshop.” Annals of Internal Medicine 172 (4): 258–271. doi: 10.7326/M19-3199. [DOI] [PubMed] [Google Scholar]
- Nelson Heidi D., Cantor Amy, Wagner Jesse, Jungbauer Rebecca, Fu Rongwei, Kondo Karli, Stillman Lucy, and Quiñones Ana. 2020. “Effectiveness of Patient Navigation to Increase Cancer Screening in Populations Adversely Affected by Health Disparities: A Meta-Analysis. “ Journal of General Internal Medicine. doi: 10.1007/s11606-020-06020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peipins LA, Soman A, Berkowitz Z, and White MC. 2012. “The Lack of Paid Sick Leave as a Barrier to Cancer Screening and Medical Care-seeking: Results from the National Health Interview Survey.” BMC Public Health 12 (1):1-. doi: 10.1186/1471-2458-12-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi N, Xia Y, Inadomi JM, Kwon SC, Trinh-Shevrin C, and Liang PS. 2019. “Disparities in Colorectal Cancer Screening in New York City: An Analysis of the 2014 NYC Community Health Survey.” Cancer Medicine 8:2572–2579. doi: 10.14309/00000434-201710001-00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauscher GH, Johnson TP, Cho YI, and Walk JA. 2008. “Accuracy of Self-reported Cancer-screening Histories: A Meta-analysis.” Cancer Epidemiology Biomarkers & Prevention 17 (4):748–757. doi: 10.1158/1055-9965.EPI-07-2629. [DOI] [PubMed] [Google Scholar]
- Rubin DB 1987. Multiple Imputation for Nonresponse in Surveys. New York, NY: Wiley. [Google Scholar]
- San Francisco Department of Public Health. 2017. “Healthy San Francisco Annual Report 2016–17.” San Francisco, CA: San Francisco Department of Public Health. https://healthysanfrancisco.org/wp-content/uploads/2016-17%20HSF%20Annual%20Report.pdf [Google Scholar]
- San Francisco Department of Public Health. 2019. “San Francisco Community Health Needs Assessment 2019.” San Francisco, CA: San Francisco Department of Public Health. https://www.sfdph.org/dph/hc/HCAgen/2019/May%207/CHNA_2019_Report_041819_Stage%204.pdf [Google Scholar]
- Sauer AG, Siegel RL, Jemal A, and Fedewa SA. 2019. “Current Prevalence of Major Cancer Risk Factors and Screening Test Use in the United States: Disparities by Education and Race/Ethnicity.” Cancer Epidemiology Biomarkers & Prevention 28 (4):629–642. doi: 10.1158/1055-9965.EPI-18-1169. [DOI] [PubMed] [Google Scholar]
- Sentell TL, Tsoh JY, Davis T, Davis J, and Braun KL. 2015. “Low Health Literacy and Cancer Screening Among Chinese Americans in California: A Cross-sectional Analysis.” BMJ Open 5 (1):1–9. doi: 10.1136/bmjopen-2014-006104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro JA, Klabunde CN, Thompson TD, Nadel MR, Seeff LC, and White A. 2012. “Patterns of Colorectal Cancer Test Use, Including CT Colonography, in the 2010 National Health Interview Survey.” Cancer Epidemiology Biomarkers & Prevention 21 (6):895–904. doi: 10.1158/1055-9965.EPI-12-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shariff-Marco S, Breen N, Stinchcomb DG, and Klabunde CN. 2013. “Multilevel Predictors of Colorectal Cancer Screening Use in California.” The American Journal of Managed Care 19 (3):205–216. doi:83985. [PMC free article] [PubMed] [Google Scholar]
- Shih YC, Elting LS, and Levin B. 2008. “Disparities in Colorectal Screening Between US-born and Foreign-born Populations: Evidence from the 2000 National Health Interview Survey.” Journal of Cancer Education 23 (1):18–25. doi: 10.1080/08858190701634623. [DOI] [PubMed] [Google Scholar]
- Shneyderman Y, Rutten LJ, Arheart KL, Byrne MM, Kornfeld J, and Schwartz SJ. 2016. “Health Information Seeking and Cancer Screening Adherence Rates.” Journal of Cancer Education 31 (1):75–83. doi: 10.1007/s13187-015-0791-6. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, and Jemal A. 2017. “Colorectal Cancer Statistics, 2017.” CA: A Cancer Journal for Clinicians 67 (3):177–193. doi: 10.3322/caac.21395. [DOI] [PubMed] [Google Scholar]
- Singh GK, and Jemal A. 2017. “Socioeconomic and Racial/Ethnic Disparities in Cancer Mortality, Incidence, and Survival in the United States, 1950–2014: Over Six Decades of Changing Patterns and Widening Inequalities.” Journal of Environmental and Public Health 2017. doi: 10.1155/2017/2819372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services and Office of Disease Prevention and Health Promotion. 2012. “Healthy People 2020.” Washington, DC: U.S. Department of Health and Human Services. https://www.healthypeople.gov/2020/data-search/Search-the-Data#objid=4054 [Google Scholar]
- U. S. Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW Jr., Garcia FAR, et al. 2016. “Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement.” JAMA 315 (23):2564–75. doi: 10.1001/jama.2016.5989. [DOI] [PubMed] [Google Scholar]
- White IR, Royston P, and Wood AM. 2011. “Multiple Imputation Using Chained Equations: Issues and Guidance for Practice.” Statistics in Medicine 30 (4):377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- Wilcox ML, Acuna JM, Rodriguez de la Vega P, Castro G, and Madhivanan P. 2015. “Factors Associated with Compliance of Blood Stool Test and Use of Colonoscopy in Underserved Communities of North Miami-Dade County, Florida.” Journal of Health Care for the Poor and Underserved 26 (4):1319–1335. doi: 10.1353/hpu.2015.0114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


