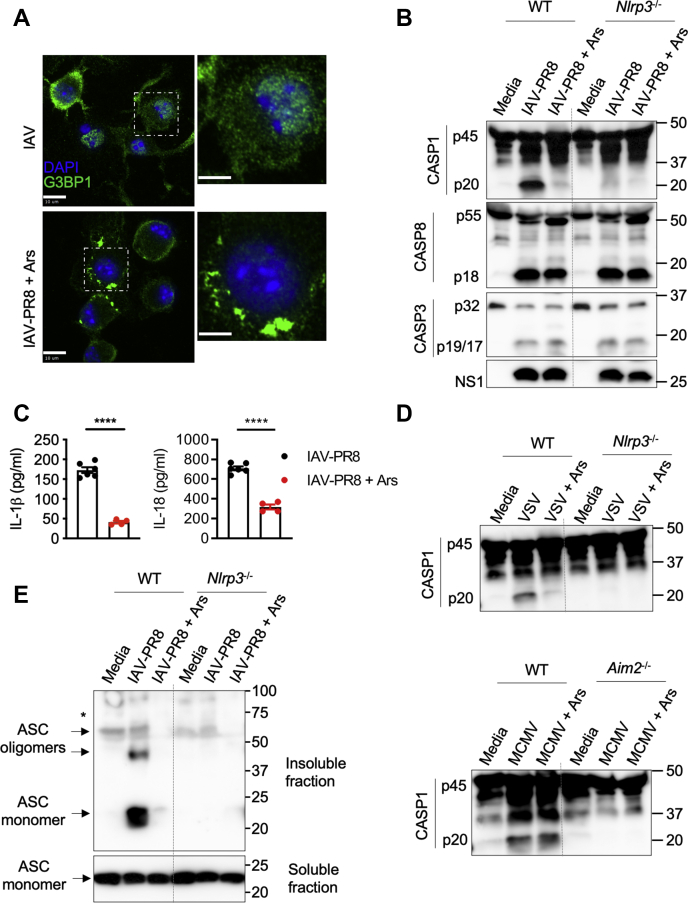
Figure 2.
Stress granule formation inhibits influenza-induced NLRP3 inflammasome activation by restricting its assembly.A, confocal microscopy imaging of bone marrow–derived macrophages (BMDMs) infected with influenza A virus (IAV)–PR8 and IAV–PR8 (MOI 20) followed by arsenite (Ars) treatment at 7 h of infection (IAV + Ars), stained for G3BP1 and DAPI. The scale bars represent 10 μm (whole image) and 5 μm (magnified image). Representative images (n = 3). B, immunoblot analysis of caspase-1 (CASP1) cleavage (pro-CASP1 (p45) and cleaved CASP1 (p20)), CASP8, and CASP3 in WT and Nlrp3−/− BMDMs infected with IAV–PR8 or IAV–PR8 + Ars (MOI 20). Representative blots (n > 3). C, ELISA measurement of IL-1β and IL-18 in BMDMs infected with IAV–PR8 or IAV–PR8 + Ars. ∗∗∗∗p < 0.0001 (unpaired two-sided t test; n > 3). Data are the mean ± SEM. D, immunoblot analysis of CASP1 cleavage in WT and Nlrp3−/− or Aim2−/− BMDMs infected with vesicular stomatitis virus (VSV) or murine cytomegalovirus (MCMV) with or without Ars treatment at 7 h of infection. Representative blots (n = 2). E, immunoblot analysis of ASC oligomerization from soluble and insoluble fractions in WT and Nlrp3−/− BMDMs infected with IAV–PR8 or IAV–PR8 + Ars (MOI 20). Representative blots (n = 3). The asterisk (∗) indicates nonspecific bands. NLRP3, nucleotide-binding oligomerization domain-like receptor with a pyrin domain 3; PR8, Puerto Rico/8/34.