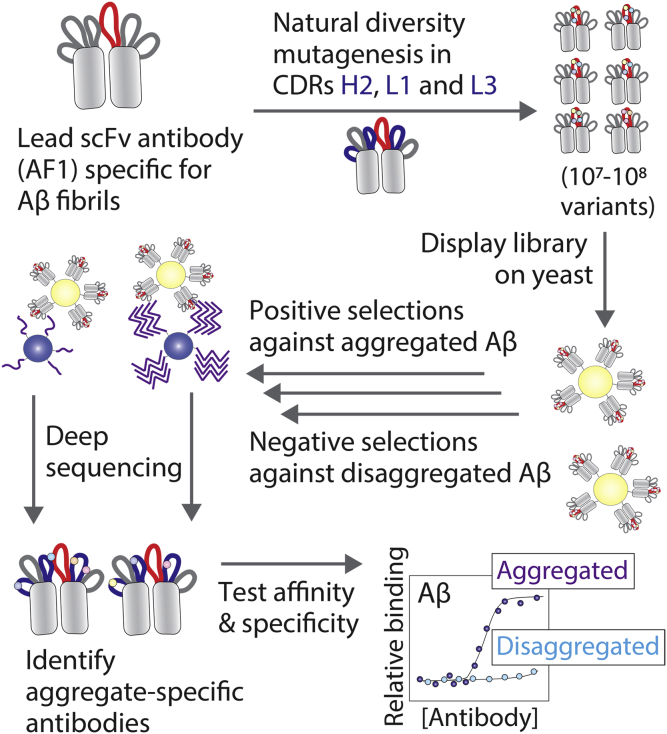
Figure 1.
Proposed method for systematically maturing the affinity and specificity of Aβ amyloid antibodies. A lead single-chain antibody fragment (scFv) specific for Aβ fibrils was mutated by targeting solvent-exposed and naturally diverse sites in three complementarity-determining regions (CDRs), including heavy-chain CDR2 (H2) and light-chain CDRs 1 (L1) and 3 (L3). The library was displayed on yeast and sorted negatively against disaggregated Aβ and positively against aggregated Aβ using magnetic-activated cell sorting. The enriched libraries were subjected to deep sequencing, and clones with mutations predicted to be favorable were evaluated in terms of their affinities and conformational specificities.