Abstract
Thrombotic thrombocytopenic purpura (TTP) is a rare hematologic disorder characterized by thrombotic microangiopathy. Neurologic symptoms are frequently seen in its presentation and the most common finding on neuroimaging of TTP is posterior reversible encephalopathy syndrome (PRES). Acute strokes, hemorrhages and atypical PRES are uncommonly seen. Our case reports the clinical and imaging details of a young male patient with TTP and Sjogren's syndrome, who made a complete recovery after aggressive plasmapheresis and immunosuppressive therapy with resolution of the imaging findings of PRES on follow up brain MR imaging. We briefly review the literature for the spectrum of imaging findings that can be seen on brain MRI with TTP.
Keywords: Thrombotic thrombocytopenic purpura, Posterior reversible encephalopathy syndrome, Atypical PRES, Microhemorrhages, MRI, Sjogren's syndrome
Introduction
Thrombotic thrombocytopenic purpura (TTP) is a rare hematologic disorder with a classic pentad of thrombocytopenia, hemolytic anemia, neurologic symptoms, fever and renal failure, characterized pathologically by microvascular thrombosis. Abnormally large Von Willebrand factor multimers in the plasma lead to microvascular thrombosis at multiple organ sites, due to deficiency of ADAMTS13, a cleaving protease. Our case highlights findings of atypical posterior reversible encephalopathy syndrome and multiple microhemorrhages on brain MRI (magnetic resonance imaging) with resolution of the signal abnormalities on follow up after plasmapheresis and immunosuppressive therapy.
Case
A 35-year-old male presented to the ED with acute onset confusion and transient tingling in his left arm which resolved by the time he was brought to the ED. A CT (computed tomography), head and CTA (computed tomographic angiography) of the head and neck was performed which was negative with no brain parenchymal or vascular abnormalities seen. The patient was subsequently discharged, only to represent 2 weeks later with fever, headache and altered mental status with waxing and waning of his attention. On neurologic examination, patient was delirious however rest of the exam was non–focal. There was diffuse petechial rash on his extremities. Laboratory evaluation was remarkable for platelets of 7000/mcl, hemoglobin of 10.6 g/dL, a large drop from baseline of 15.6 g/dL, LDH of 817 U/L, haptoglobin <10 mg/dL, concerning for hemolytic anemia and thrombocytopenic thrombotic microangiopathy. Renal function was normal with serum creatinine of 1.0 mg/dL and calculated GFR > 60 mL/min/1.73 m2. Findings were highly concerning for TTP and patient was admitted to the intensive care unit (ICU) with initiation of high dose steroids and plasmapheresis. ADAMTS13 activity assay was performed which was less than 3%, confirming TTP (a value less than 10% activity is considered severely deficient).
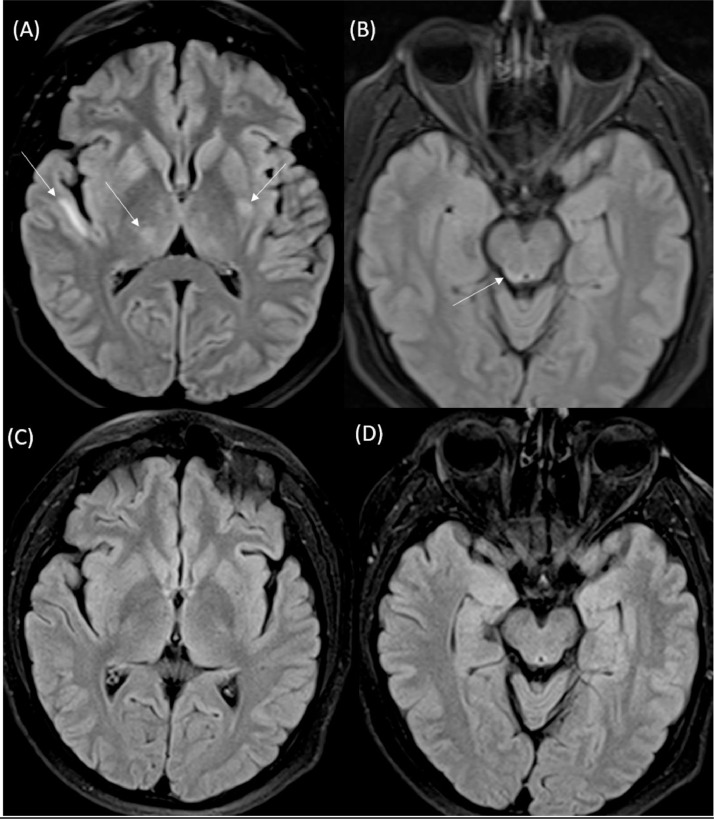
A MRI brain study was performed without and with intravenous contrast on a 1.5T scanner on day 1 of the hospital admission. The brain MRI demonstrated multiple patchy areas of T2 and FlAIR (fluid attenuated inversion recovery) hyperintense signal abnormalities in the right perisylvian white matter, right thalamus, left lentiform and right aspect of midbrain (Fig. 1). On SWI (susceptibility weighted imaging) sequence, there were foci of low signal in these regions consistent with microhemorrhages (Fig. 2). No abnormal enhancement was noted on post contrast sequences. Review of his prior CT imaging demonstrated parotid calcifications and cysts (Fig. 3), and the concern for Sjogren's syndrome was brought forth by the radiologist. Differential considerations based on MRI included atypical PRES related to TTP or viral encephalitis. To rule out possibility of encephalitis, CSF (cerebrospinal fluid) analysis with a lumbar puncture was performed. There was no evidence of infection -CSF was clear, cell count of 2 WBCs/dL, Glucose of 67 mg/dL (normal range of 40-70 mg/dL), Protein of 48 mg/dL (normal range of 15-45 mg/dL). CSF culture had no growth and viral PCR and anti–body panel (for West Nile, HSV, VZV and enteroviruses) were negative. Immunologic lab tests were performed and was positive for Sjogren's syndrome with a positive SS-A anti–body test.
Fig. 1.
Axial FLAIR MRI (A and B) performed on day 10 of admission demonstrates multifocal hyperintense signal abnormalities in the right perisylvian white matter, right thalamus, left lentiform and right midbrain (white arrows). On follow up MRI, performed 4 weeks later, while patient was on plasmapheresis and immunosuppressive therapy, selected axial FLAIR images (C and D) at the same levels show that there was complete resolution of the previously seen abnormal signal.
Fig. 2.
SWI images from the initial MRI brain study demonstrate multiple tiny foci of low signal (white arrows) which corresponded to areas of FLAIR signal abnormality seen in Fig. 1, consistent with microhemorrhages.
Fig. 3.
Axial T2 image from patient's brain MRI (A) and Axial CT image (B) from a study at time of presentation demonstrate bilateral parotid tiny cysts (black arrows) and foci of calcification (white arrows), for which concern about Sjogren's syndrome was brought forth and subsequently confirmed with a positive serologic SS-A antibody test.
Subsequently, the patient had a prolonged hospital course of 5 weeks with multiple courses of plasmapheresis and weekly Rituximab therapy for 4 doses with eventual normalization of his platelet levels and resolution of hemolytic anemia. ADAMTS13 activity normalized after this prolonged immunosuppressive therapy. His mental status also gradually improved over the course of the hospitalization and a follow up MRI performed 4 weeks later demonstrated complete resolution of previously seen FLAIR signal abnormalities (Fig. 1 C and D). Patient was finally discharged on oral steroids with plan for steroid taper. On 3 months follow in the outpatient clinic, the patient was now off oral steroids was doing well symptomatically with normal lab work.
Discussion
TTP is a rare entity which can be potentially lethal if not recognized and treated early underscoring its clinical significance. Deficiency of ADAMTS13 protease is the central etio-pathologic factor [1]. The 2 major types of TTP are- 1. Congenital TTP, which is due to ADAMTS13 deficiency and 2. Immune mediated TTP, which is due to presence of ADAMTS13 autoantibodies. Immune mediated TTP typically occurs in adulthood, median age of onset of fourth decade and more common in females to male with a ratio of 2 to 3:1. Immune mediated TTP can either be primary or idiopathic or secondary to a predisposing condition. Predisposing conditions include autoimmune diseases, malignancy, infection, drugs and pregnancy. Amongst the autoimmune conditions, SLE (systemic lupus erythematosus) is the commonest, although many others autoimmune disorders including Sjogren's syndrome as seen in our case, have been noted in association with TTP [2,3].
Clinical evaluation: The diagnosis of TTP is made primarily based on a combination of clinical and laboratory findings. Although, the classic clinical pentad of thrombocytopenia, hemolytic anemia, neurologic symptoms, fever and renal failure has been described for TTP, the diagnosis remains challenging due to variability in clinical features, overlap with other thrombotic microangiopathies and limited availability of the ADAMTS13 test [4]. The diagnosis of TTP is suspected when there are findings of microangiopathic hemolytic anemia and thrombocytopenia which are universally present in all cases of TTP. Neurologic symptoms and renal failure which are due to end organ damage occur variably. Although not gold standard, the ADAMTS13 activity and ADAMTS13 inhibitor assays have been used for confirmation of diagnosis of TTP in clinically suspected cases. Scoring systems incorporating clinical and laboratory findings like the PLASMIC score have been used in prediction of TTP [5].
Imaging evaluation: Up to 90% patients of TTP have been found to display neurologic symptoms [6]. Neuroimaging in TTP can be performed with either CT or MRI brain imaging. CT is less sensitive in detection of ischemia and microhemorrhages compared to MRI. Posterior reversible encephalopathy syndrome (PRES) is the most common imaging manifestation seen in patients with TTP on MR imaging [7]. Typical PRES involves the posterior aspects of the supratentorial brain parenchyma ie the parietal and occipital lobes, with classic appearance of white matter edema on both CT and MR imaging of the brain [8]. PRES is considered atypical when there is involvement of either atypical locations, restricted diffusion or presence of hemorrhage [9]. Atypical locations include frontal or temporal lobes, deep grey matter or the infratentorial brain parenchyma. The most important criteria for calling a signal alteration as PRES is its reversibility which can be confirmed only with follow up imaging.
The most widely accepted hypothesis for PRES is of vascular dysregulation, with rapid increase in blood pressure that leads to development of edema in the posterior parieto-occipital lobes as the posterior circulation has a sparse sympathetic innervation compared to the anterior carotid circulation. Pathophysiology involving systemic inflammation and endothelial dysfunction have also been considered as PRES occurs in multiple inflammatory conditions such as sepsis, preeclampsia and auto immune disorders [10]. The pathophysiology for PRES and other neuroimaging findings in TTP are not well elucidated but probably relate to microvascular thrombosis with subsequent perfusional disturbances and inflammation that accompanies TTP.
Acute infarcts and hemorrhages are less frequently seen in TTP. In most patients, the brain MRI findings in TTP are completely reversible, although there are isolated reports of persistent small infarcts seen on MRI after clinical recovery [11]. There is also a case report where a large acute infarct was seen from large vessel thrombosis in TTP. Presence of hemorrhage makes PRES more severe, although there are no large studies comparing whether presence of infarcts and hemorrhages are associated with worse outcomes in TTP. In our case, the brain parenchymal signal abnormalities were not in the typical parieto-occipital distribution, with involvement of basal ganglia, midbrain, thalamus and temporal white matter. Presence of microhemorrhages is also uncommonly reported. Both ischemia and hemorrhage are potentially possible in this disorder to manifest in the brain based on the hematologic alterations of thrombocytopenia and microvascular thrombosis seen in this disorder.
Conclusion
Atypical PRES and microhemorrhages can be seen on MRI brain imaging in TTP. Prompt diagnosis and treatment of TTP is important to ensure complete recovery and a good prognosis. Imaging findings are typically reversible after successful treatment and can be used for follow-up surveillance. Auto-immune diseases can be associated with TTP and should be suspected both clinically and on imaging. In our case, Sjogren's disease was suspected due to parotid abnormalities on imaging.
Patient consent
There has been anonymization of any identifiable personal patient information. Patient consent has been obtained.
Footnotes
Acknowledgments: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Competing interest: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Levy GG, Nichols WC, Lian EC, Foroud T, McClintick JN, McGee BM. Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature. 2001;413(6855):488–494. doi: 10.1038/35097008. [DOI] [PubMed] [Google Scholar]
- 2.Swisher KK, Lewis QF, James JA, Hovinga JAK, Lämmle B, Terrell DR. The Frequency of Rheumatic Disease Autoantibodies in Patients with ADAMTS13-Deficient Thrombotic Thrombocytopenia Purpura (TTP) Blood. 2007;110(11):2090. [Google Scholar]
- 3.Noda M, Kitagawa M, Tomoda F, Iida H. Thrombotic thrombocytopenic purpura as a complicating factor in a case of polymyositis and Sjogren's syndrome. Am J Clin Pathol. 1990;94(2):217–221. doi: 10.1093/ajcp/94.2.217. [DOI] [PubMed] [Google Scholar]
- 4.Chiasakul T, Cuker A. Clinical and laboratory diagnosis of TTP: an integrated approach. Hematology Am Soc Hematol Educ Program. 2018;2018(1):530–538. doi: 10.1182/asheducation-2018.1.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wynick C, Britto J, Sawler D, Parker A, Karkhaneh M, Goodyear D. Validation of the Plasmic Score for Predicting ADAMTS13 Activity < 10% in Patients Admitted to Hospitals in Alberta with Suspected Thrombotic Thrombocytopenic Purpura. Blood. 2019;134(Supplement_1):2379. doi: 10.1016/j.thromres.2020.09.012. [DOI] [PubMed] [Google Scholar]
- 6.Meloni G, Proia A, Antonini G, De Lena C, Guerrisi V, Capria S. Thrombotic thrombocytopenic purpura: prospective neurologic, neuroimaging and neurophysiologic evaluation. Haematologica. 2001;86(11):1194–1199. [PubMed] [Google Scholar]
- 7.Burrus TM, Wijdicks EF, Rabinstein AA. Brain lesions are most often reversible in acute thrombotic thrombocytopenic purpura. Neurology. 2009;73(1):66–70. doi: 10.1212/WNL.0b013e3181aaea1b. [DOI] [PubMed] [Google Scholar]
- 8.Raman R, Devaramane R, Jagadish GM, Chowdaiah S. Various Imaging Manifestations of Posterior Reversible Encephalopathy Syndrome (PRES) on Magnetic Resonance Imaging (MRI) Pol J Radiol. 2017;82:64–70. doi: 10.12659/PJR.899960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKinney AM, Short J, Truwit CL, McKinney ZJ, Kozak OS, SantaCruz KS. Posterior Reversible Encephalopathy Syndrome: Incidence of Atypical Regions of Involvement and Imaging Findings. American Journal of Roentgenology. 2007;189(4):904–912. doi: 10.2214/AJR.07.2024. [DOI] [PubMed] [Google Scholar]
- 10.Bartynski WS. Posterior reversible encephalopathy syndrome, part 2: controversies surrounding pathophysiology of vasogenic edema. AJNR Am J Neuroradiol. 2008;29(6):1043–1049. doi: 10.3174/ajnr.A0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gruber O, Wittig L, Wiggins CJ, von Cramon DY. Thrombotic thrombocytopenic purpura: MRI demonstration of persistent small cerebral infarcts after clinical recovery. Neuroradiology. 2000;42(8):616–618. doi: 10.1007/s002340000348. [DOI] [PubMed] [Google Scholar]