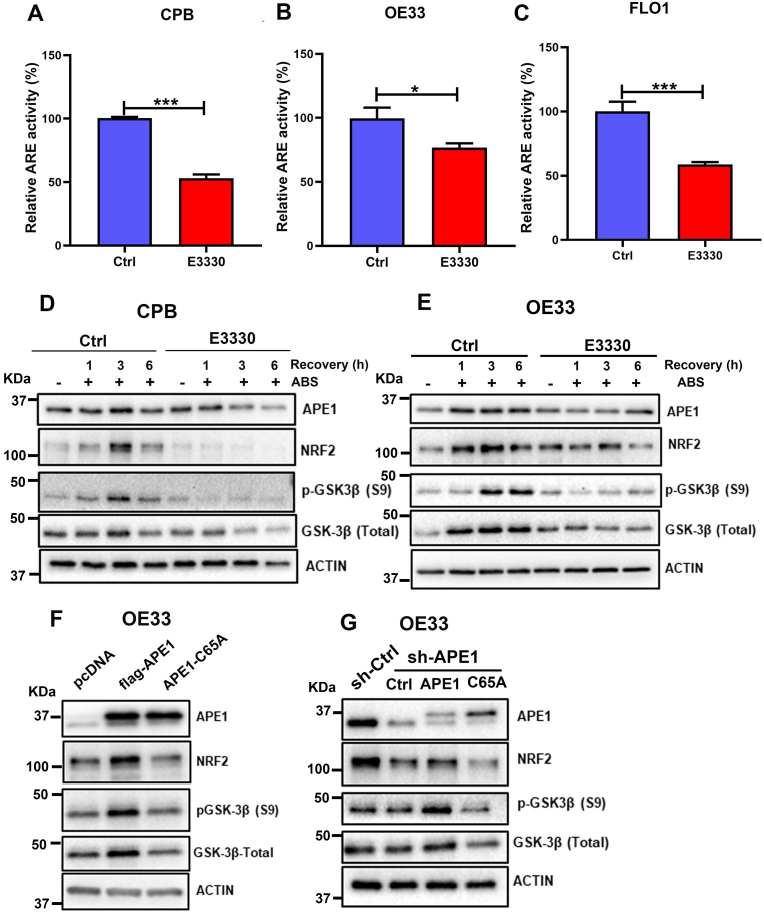
Fig. 7.
APE1 redox function was required for GSK-3β-mediated APE1 regulation of NRF2. (A–C) CPB, OE33 and FLO1 cells were pre-treated with or without E3330, an APE1 redox inhibitor for 24 h. Cells were then transfected with PGL3-NRF2-ARE-Luc and β-gal with or without E3330 for another 24 h. The relative ARE luciferase reporter activity was measured and is shown as a percentage relative to controls (set as 100%). Values are mean ± SD of three independent experiments. *P < 0.05; ***P < 0.001. (D and E) CPB and OE33 cells were pre-treated with or without E3330 (100 μM) for overnight followed by treatment with ABS (100 μM) for 20 min and recovery in full media for 1, 3 and 6 h post-treatment with or without E3330. Western blot analyses were used to determine the levels of APE1, NRF2, GSK-3β (Total) and p-GSK-3β (S9). β-actin was used as a loading control. (F) OE33 cells were transfected with wild type APE1 with the flag (flag-APE1) or mutant APE1 C65A (APE1-C65A, an APE1 redox mutant). Western blot was applied for the levels of NRF2, APE1, GSK-3β (Total) and pGSK-3β (S9). (G) OE33 cells with stable knockdown of APE1 using sh-APE1 were reconstituted with wild type APE1 (APE1) or mutant APE1 C65A (C65A). Western blot was used to analyze the protein levels of NRF2, APE1, GSK-3β (Total) and pGSK-3β (S9).