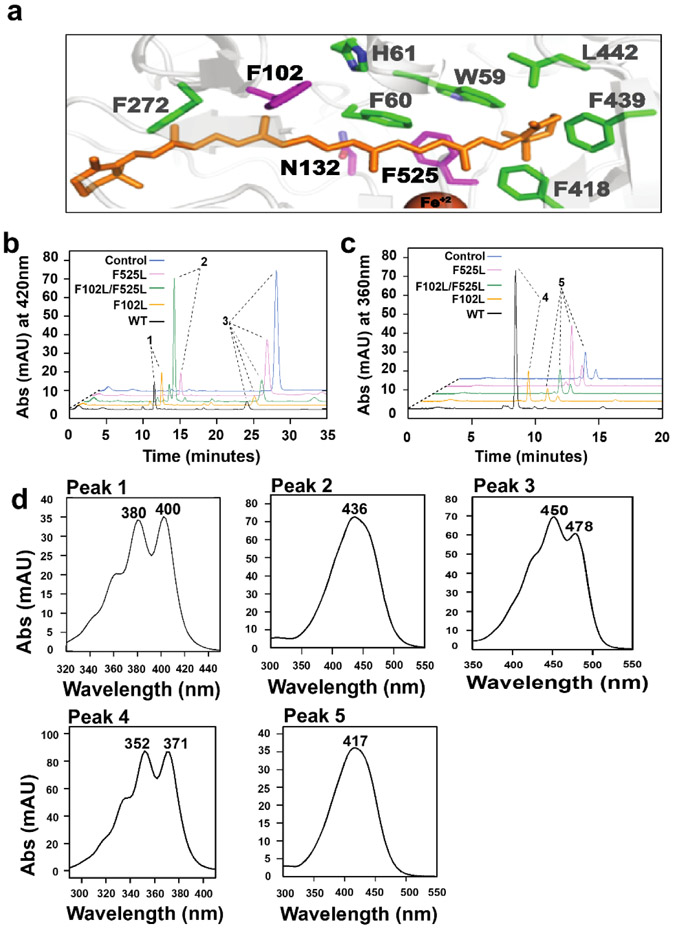
Figure 5.
BCO2 possesses a bipartite substrate binding cavity which discriminates between carotenoids and apocarotenoids. a) Close up view of the modeled substrate binding cavity of BCO2 with bound β-carotene (orange). The cavity is lined with aromatic amino acid side residues (green) which are conserved between BCO1 and BCO2. Mutated amino acid residue F102, N132 and F525 are highlighted in magenta. b) HPLC traces at 420 nm of enzyme assays of wild type and mutant mBCO2 variants incubated with meso-zeaxanthin. In control experiments, the substrate was incubated with maltose binding protein c) HPLC traces at 360 nm of enzyme assays of wild type and mutant BCO2 variants incubated with 3-hydroxy-β-apo-12’-carotenal. In control experiments, the substrate was incubated with maltose binding protein. d) UV-visible spectra of major products’ peaks in B and C. Peak 1is 10’,10-apocarotene-dialdehyde; peak 2 is 3-β-apo-10’ carotenal, peak 3 is meso-zeaxanthin; peak 4 is 12’,10-apocarotene-dialdehyde; peak 5 is 3-hydroxy-β-apo-12’-carotenal.