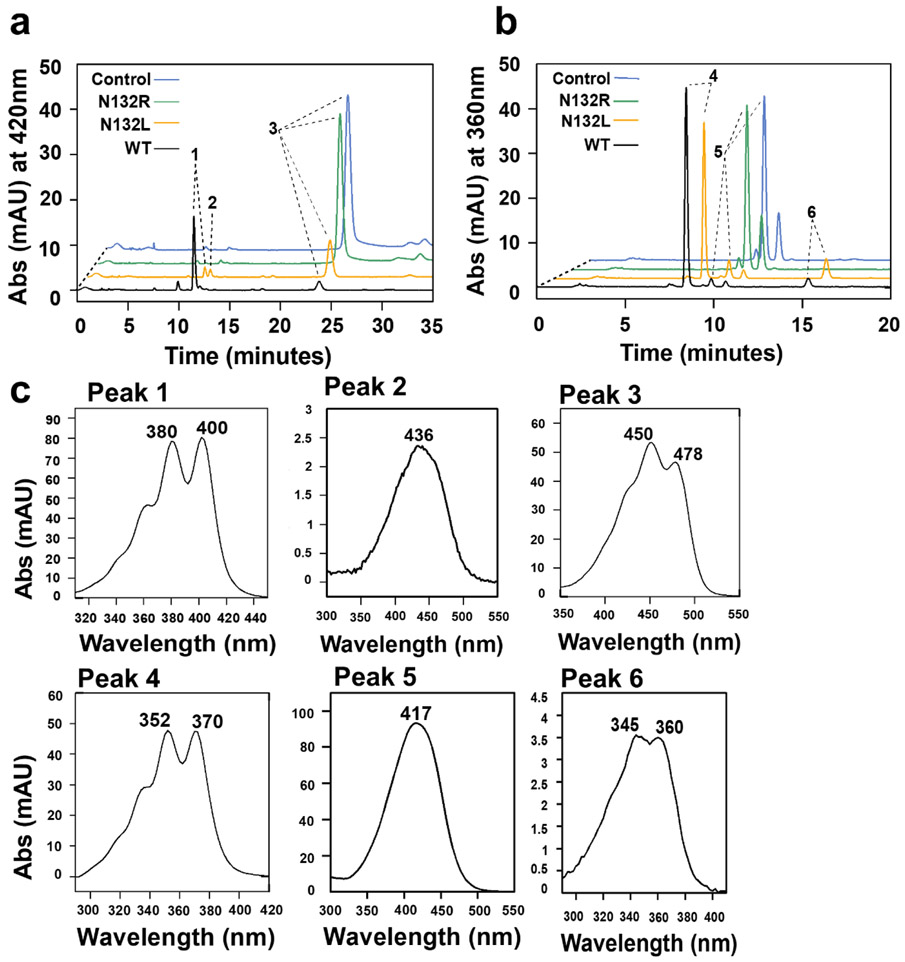
Figure 6.
Asn132 stabilizes π electron bonding in the active center of BCO2. a) HPLC traces at 420 nm of enzyme assays of wild type and mutant mBCO2 variants incubated with meso-zeaxanthin. In control experiments, the substrate was incubated with maltose binding protein b) HPLC traces at 360 nm of enzyme assays of wild type and mutant mBCO2 variants incubated with 3-hydroxy-β-apo-12’-carotenal. In control experiments, the substrate was incubated with maltose binding protein. c) UV-visible spectra of products and substrates. peak 1, 10’,10-apocarotene-dialdehyde; peak 2, beta-apo-10’-carotenal; peak 3, meso-zeaxanthin; peak 4, 12’,10-apocarotene-dialdehyde; peak 5, 3 hydroxy-β-apo-12’-carotenal; peak 6, 10’,10-apocarotene-diol.