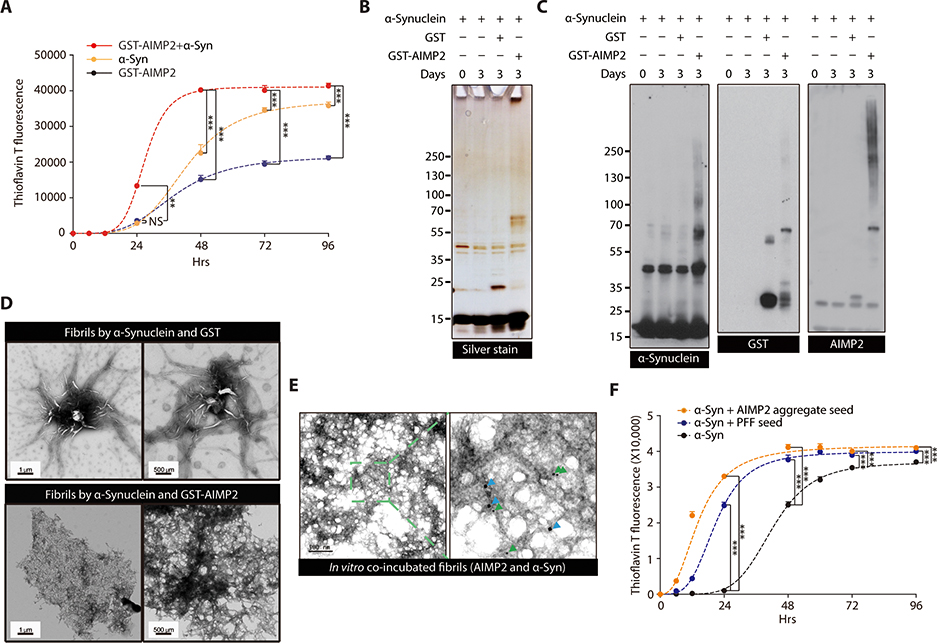
Figure 3.
α-synuclein oligomer/fibril formation is enhanced by AIMP2 in vitro
(A) ThT fluorescence assay of in vitro aggregation for recombinant α-synuclein (50 uM) and/or GST-AIMP2-FLAG (50 uM) (n = 4 per group).
(B) Silver-stained gel image of in vitro incubation samples of α-synuclein (50 uM) and/or GST-AIMP2-FLAG (50 uM).
(C) Representative immunoblots of α-synuclein or AIMP2 for in vitro incubation samples with various combinations.
(D) Ultrastructure of protein aggregates prepared from α-synuclein incubation (7 d) with either GST or GST-AIMP2-FLAG monitored by a TEM.
(E) Localization of AIMP2 and α-synuclein in protein aggregate formed by in vitro coincubation of recombinant AIMP2 and α-synuclein determined by immuno-Gold EM using 10 nm (green arrow) or 20 nm (blue arrow) nanoGold particles conjugated to anti-AIMP2 and anti-α-synuclein antibodies, respectively.
(F) ThT fluorescence assay of in vitro aggregation for recombinant α-synuclein (50 uM) alone or with small amounts of PFF (2.5 uM) or AIMP2 preformed aggregate (2.5 uM) (n = 3 per group).
Quantified data are expressed as mean ± SEM. Statistical significance was determined by ANOVA test with Tukey post-hoc analysis, **p < 0.01, and ***p < 0.001; NS: not significant.