Abstract
Under normal conditions, coronary blood flow (CBF) provides critical blood supply to the myocardium so that it can appropriately meet the metabolic demands of the body. Dogmatically, there exist several known regulators and modulators of CBF that include local metabolites and neurohormonal factors that can influence the function of the coronary circulation. In disease states such as diabetes and myocardial ischemia, these regulators are impaired or shifted such that CBF is reduced. Although functional considerations have been and continued to be well studied, more recent evidence builds upon established studies that collectively suggest that the relative roles of coronary structure, biomechanics, and the influence of cardiac biomechanics via extravascular compression may also play a significant role in dictating CBF. In this mini review, we discuss these regulators of CBF under normal and pathophysiological conditions and their potential influence on the control of CBF.
Keywords: coronary blood flow, function, structure, biomechanics, stiffness
INTRODUCTION
Coronary blood flow (CBF) provides the necessary nutrients to the cells of the heart for sustaining metabolic demand. Since the coronary vasculature transports blood to and from the heart muscle, any impairment that reduces CBF to the cardiac muscle can lead to serious pathological conditions such as heart failure. In this unique branch of the circulatory system, phasic blood flow is greatest during cardiac diastole and relies on a multitude of factors to regulate flow under normal conditions, including functional, structural, biomechanical, and extravascular influences; however, under pathological conditions, such as diabetes and myocardial ischemia, these factors go awry. The current state of coronary pathological investigations relies heavily on functional aspects segregated from other important facets, including the structure and biomechanics of the coronary circulation, comprised of both conduit vessels and the microcirculation, in part, because of the complexity in studying them in an integrated manner. Functional mediators of CBF and their interactions together have been the subject of multiple excellent recent reviews, including reviews from Muller-Delp et al. (1, 2) and Goodwill et al. (3).
The goal of this mini review is to assess the past and current state of nonfunctional regulators of CBF in disease as depicted in Fig. 1 (e.g., structural, biomechanical, and extravascular), simplistically using Poiseuille’s Law as a guide. Poiseuille’s Law describes laminar flow through a tube; therefore it can be used to estimate blood flow through a vessel:
where = flow, = change in pressure, = radius, = viscosity, and = vessel length. The largest contributor to flow is vessel radius, thus our focus will be related to that since very small changes in radius lead to large changes in blood flow. We will briefly review and explore these and other modulators of CBF in this work.
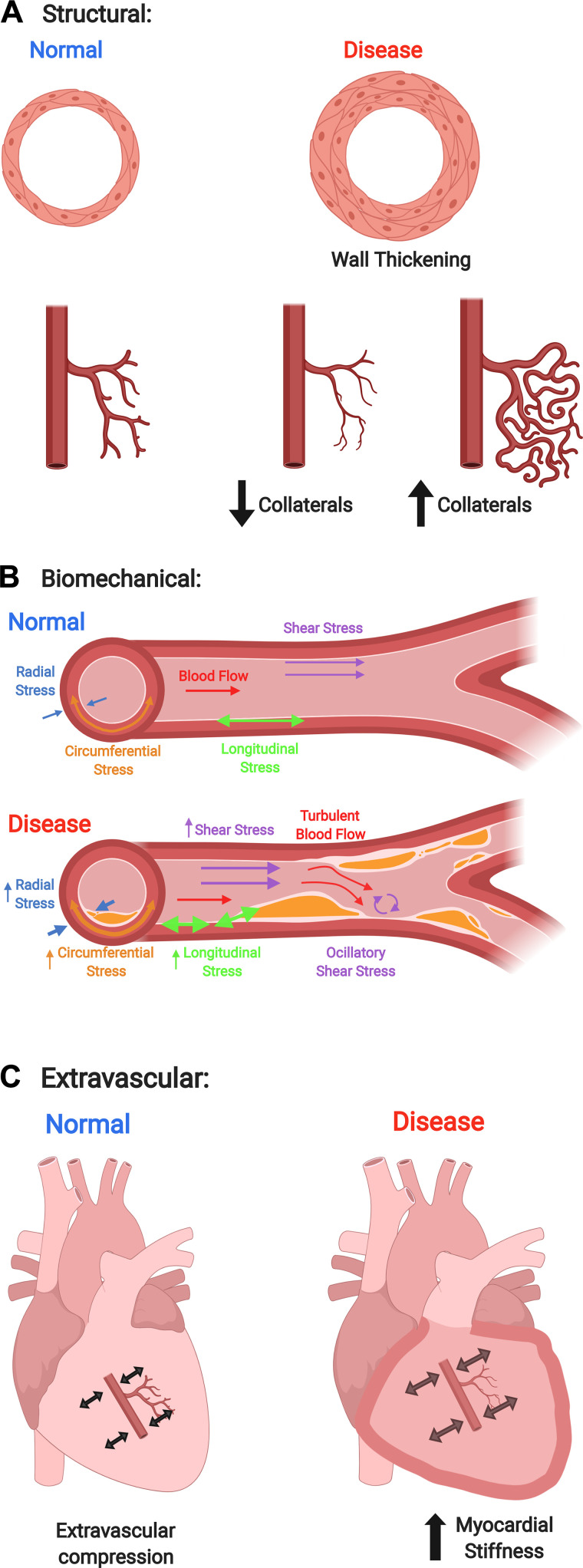
Figure 1.
Coronary blood flow (CBF) is regulated by a myriad of physiological parameters. Conventionally, studies have focused on one of these parameters, however, under pathological conditions, typically more than one of these parameters goes awry. In this figure we depict how structural (A), biomechanical (B), and extravascular (C) parameters may change during normal and pathological conditions. Typically, functional changes are due to vasodilation or vasoconstriction to maintain CBF. Structural modifications may include changes in radius, wall thickness, and vessel length. Biomechanical factors include vessel material properties (i.e., stiffness), and forces acting on the vessels (e.g., shear, longitudinal, radial, and circumferential stresses). Extravascular alterations may include changes in myocardial stiffness and changes in viscosity. The figure was created using www.BioRender.com with permission.
STRUCTURAL CONSIDERATIONS THAT INFLUENCE CORONARY BLOOD FLOW
The structure of the coronary vascular tree can be divided into conduit arteries that arise from the coronary ostia in the aorta, arterioles (resistance microvessels that are classically defined as having an internal diameter < 150 µm), capillaries, venules, and veins that collect in the coronary sinus and back to the heart (4). This parent-daughter branching pattern, such as other vascular beds, plays a role in the distribution of blood flow (4). Among structural alterations that occur in pathophysiological states are occlusive atherosclerosis and coronary microvascular remodeling. Atherosclerosis is a chronic, proinflammatory disease wherein lesions develop in large- and medium-sized arteries and can induce ischemia of organs, including the heart, brain, and extremities (5). In the heart, atherosclerotic lesions form at coronary branch points, bifurcations, curvatures, or other areas of oscillatory flow (6). Beyond plaque formation, vascular remodeling occurs at these sites mainly via extracellular matrix (ECM) changes that alter the integrity of the arterial wall, primarily from matrix metallopeptidase (MMP) activity (7, 8). Although atherosclerotic remodeling of the conduit arteries causes narrowing, a 50% stenosis, for example, has very little effect on CBF due to compensatory autoregulation of the microcirculation (9). CBF is not largely compromised until stenosis reaches ∼70% (10). In addition to severe stenosis of the coronary conduits, remodeling of the coronary microcirculation also has a potent impact on coronary flow. Our laboratory has previously reported that in both mouse and swine models of type 2 diabetes mellitus (T2DM) and metabolic syndrome (MetS), coronary resistance microvessels (CRMs) undergo inward hypertrophic remodeling associated with decreased lumen diameter, increased wall thickness, and decreased stiffness; however femoral stiffness was increased in db/db mice and the left anterior descending coronary artery (LAD) of MetS pigs had increased β stiffness index, indicated a dichotomous response to diabetes in the CRMs versus larger vessels (11, 12). Data from the Muller–Delp laboratory has demonstrated a similar remodeling phenomenon in CRMs from aging rats with increased wall/lumen ratio and decreased elastic modulus (13). In our studies, the adverse structural changes were accompanied by a decrease in CBF and coronary flow reserve (CFR), suggesting that microvascular structural remodeling may account for at least some of the deleterious ischemic events that occur in MetS and T2DM (11, 12, 14). Our laboratory has recently published that CRMs from aging mice undergo adverse remodeling in the same manner that CRMs from young, db/db mice experience (15). In fact, from these data, one could argue that diabetes imparts an accelerated aging process on the coronary microcirculation as the CRMs in db/db mice undergo adverse remodeling at an early age, similar to normal aged CRMs, that persists toward senescence. The data from our previous studies indicate that reduced coronary microvascular radius can negatively impact CBF (11, 12, 16).
Besides circumferential remodeling of the coronaries, another structural component that impacts CBF is the longitudinal branching in the coronary circulation via collateral growth. Coronary collateral growth is a compensatory mechanism that occurs during episodes of myocardial ischemia in an attempt to maintain flow to this depleted area through remodeling of small arterioles to larger arterioles and arteries (17, 18). There are multiple reviews specific to this area and its importance to CBF maintenance during myocardial ischemia (19–22).
BIOMECHANICS IN THE CORONARY CIRCULATION
When determining the role of biomechanics in CBF management, it is important to consider both the passive material properties and active mechanical forces of the coronary vasculature. Passive mechanics are the mechanical material properties of the tissue, derived from cellular and molecular structure and organization (23). An integral mechanical property of any blood vessel is the stiffness of its wall, often reported as elastic modulus. Conversely, vascular distensibility is inversely related to its stiffness (24, 25). Active mechanical components are any of the forces that act on the coronary vasculature, whether from blood flowing through the vessel or tissue surrounding the vessel contracting or relaxing. In terms of Poiseuille’s Law, both active and passive mechanics impact the ability of a vessel to alter its radius to control flow, due to passive tissue properties influencing how vessels are able to respond to alterations in active mechanical forces. Although much of the knowledge on coronary biomechanics focuses on the effects of altered forces on disease progression, it has become increasingly clearer that passive mechanics are also playing a large role in coronary flow regulation (11, 12, 26–29). Here, we will discuss what is currently known regarding coronary biomechanics and provide commentary on how mechanics are playing a role in maintenance of CBF.
Passive Mechanical Properties of the Coronary Vasculature
To examine the impacts of mechanical forces on flow through the coronary vasculature, it is important to first understand the mechanical properties inherent to the coronary circulation. Clinically, diagnostics such as MRI and ultrasound have been developed to measure vessel stiffness (24, 25, 30, 31). Alterations in arterial compliance in both large systemic arteries and large coronary conduit arteries have been linked with aging, hypertension, smoking, and coronary artery disease (CAD) (32–37). Importantly, increased conduit vessel stiffness in disease is often associated with decreased flow or endothelial dysfunction (28, 29). Similarly, increased stiffness in large arteries (carotid or aorta) leads to decreased flow within the coronary circulation (38–43); however, these concepts may not be universally applied as stiffness can also be compounded by alterations in coronary radius elicited by its own structure, function, and biomechanics, and as discussed earlier, radius provides the largest contribution to coronary flow according to Poiseuille’s Law. Data from our laboratory show that the diabetic coronary microvasculature has reduced radius (diameter) and reduced stiffness and the microcirculation has reduced flow (11, 12, 16). In the setting of diabetes, it may be that the reduction in diameter drives flow more than stiffness, which could be reduced in a compensatory effort to mitigate further reductions in CBF. Other aging data also implicates coronary biomechanics in influencing CBF. In two independent studies from the Muller–Delp and Trask laboratories, the passive diameters of CRMs between normal young and aged rodents were similar, but both studies reported a reduction in stiffness of the aged CRMs (13, 15). In the latter study, this was associated with a reduction in hyperemic CBF (15). Although one would theoretically predict that reduced CRM stiffness should improve CBF if diameters were similar, perhaps in the setting of aging, the reported impaired coronary dilator function, as reviewed elsewhere (1, 3), drives CBF more than alterations of radius/diameter and biomechanics. Nonetheless, although teasing apart these integrated factors is difficult, these observations point to a clear association between vessel stiffness and CBF through the vessel, and further studies in this important area are warranted. Finally, it is also important to note that material properties of the coronary vessels vary greatly depending on the type of vessel. Through post hoc analysis of data collected in our laboratory on both mouse and pig coronary vessels, larger conduit arteries (aorta and left anterior descending coronary) are inherently less stiff than the resistance microvessels (11, 12, 26). The reasons for this are currently unclear, but, collectively, these data may begin to give insight into how the mechanical properties of any blood vessel have an impact on CBF. It is intriguing to speculate that augmented microvascular stiffness as compared with macrovascular stiffness may result from differences in ECM, vascular smooth muscle cell (VSMC), and/or location of applied forces on the vessel (e.g., coronary epicardial conduit arteries are not subject to extravascular compression that occurs during the cardiac cycle). Perhaps in the coronary vascular tree, the microcirculation needs to be more stiff than upstream conduit arteries to have the mechanical integrity to withstand cardiac compression. These concepts will be discussed in the following sections.
Pulsatility and Waves (Origin and Reflections)
One of the most important drivers of coronary flow is derived from the active force the myocardium exerts on the coronary vasculature (44–46). This force, often referred to as extravascular compression, is the compression of embedded coronary vessels as the heart contracts (47–49). As the myocardium contracts during systole, vessels are compressed and flow is diminished. When the myocardium relaxes during diastole, the coronaries expand and flow through the vessel is restored, particularly in the left ventricle (47–49). Therefore, flow through the coronary vasculature is at a maximum during diastole and at a minimum during systole.
The collapsibility of coronary vessels (as a function of myocardial force) brings about the vascular waterfall (or Starling Resistor) analogy as a potential descriptor of CBF. In their studies of CBF during systole, Downey and Kirk (50) describe the mechanism in detail. In brief, the waterfall analogy refers to blood flowing beyond a partially collapsed portion of a vessel where flow is independent of inlet pressure, or the height of the “waterfall” where fluid beyond the fall flows freely. This proposed mechanism is of particular interest because it provides a potential explanation for how contraction of the myocardium and resultant compressive force limits blood flow (50). Although others provide evidence of the existence of a vascular waterfall in the coronary circulation (51–53), there continues to be debate on whether or not the proposed analogy fully describes the true oscillatory behavior coronary flow (46, 54, 55). Some argue that although it is clear the description of the vascular waterfall is useful in modeling CBF, certain aspects of flow through collapsible blood vessels (such as variable vessel resistance, geometry, and the inherent impact of the myocardium as a pump driving flow) are neglected in the analogy.
Waves in coronary blood flow arise from forces acting upon the coronary circulation, or the active component of coronary biomechanics. Reflections in these waves occur whenever there is a change in the vessel impedance (a measure of both the vessels compliance and cross-sectional area). Both the formation and impact of flow wave reflections in the coronary tree have been questioned, and there are very few studies on the topic as a result. Typically, the constant wall impedance and short length of coronary vessels has led many to believe that wave reflections are insignificant in the formation and driving of coronary flow (56, 57). However, recent studies (both computational and experimental) point to potential significant effects of wave reflections in coronary flow, where reflections may actually enhance early diastolic flow (56, 57).
As with many other biomechanical measurements, arterial wave reflections are often measured in the aorta and other large vessels in the clinic and not the coronary vasculature. Therefore, many clinical studies that link arterial stiffness and wave reflections with CAD and other heart-related disease do not focus on wave dynamics in the coronary arteries (37, 58). Because of this, there is little to no clinical evidence of the impact of wave reflections on coronary flow and underlying disease, although it is apparent that the topic requires further studying.
Endocardial versus Epicardial Mechanics
As coronary vessels can be both endocardial and epicardial, it is important to note that the passive mechanics of the vessels may change based on their location within myocardial tissue and how they are exposed to active forces derived from cardiac contraction. Although some have reported marked differences in endocardial and epicardial coronary flow profiles (59–61), others report much smaller differences (62–64). These apparent small differences in flow profiles between the two vessel types are surprising, especially as it is well established that embedded endocardial coronary vessels are subjected to significantly greater compressive forces with contraction of the myocardium (65).
When considering the flow profiles of endocardial and epicardial coronary vessels, it is important to note that the large differences in force the vessels are exposed to likely cause differences in vessel structure, and therefore passive biomechanical characteristics. Specifically, differences in coronary stiffness and distensibility have been long debated. Of particular interest is where overall coronary capacitance is derived, that is, whether endocardial or epicardial coronary vessels may act as a type of capacitor, where changes in blood volume are accompanied by smaller changes in pressure. Although there is evidence of nonnegligible epicardial coronary capacitance (60, 66–68), more recent evidence points to overall coronary capacitance likely being derived from endocardial arteries (67, 69, 70). Interestingly, these observations are paired with differences in the distribution of endocardial and epicardial coronary resistances (71), which is hypothesized to play a role in the development of coronary capacitance.
Cellular versus Extracellular Determinants of CBF
To truly understand the integrated biomechanics regulating CBF, we must consider the possible individual contributors. Classically, the ECM has been a well-studied contributor to vascular stiffness. There are a plethora of data pointing to a positive association between ECM accumulation and vascular stiffness, including in the coronary conduit arteries, often precipitated by an accumulation of fibrillar collagens. On the contrary, not all vessels become stiff in disease. We showed that CRMs from T2DM mice and MetS pigs are actually less stiff with augmented elastin expression (11, 12). The Wegenseil and Mecham groups have extensively analyzed the roles of elastin and other elastic fibers in aortic biomechanics. There are a number of reviews that discuss ideas such as vascular ECM development and organization and its role in general arterial mechanics (72), and more specifically, the importance of elastic fibers in aortic mechanical development (73, 74). How these specifically apply to the coronary macro- and microcirculation are just beginning to be understood.
More recent data point to the vascular smooth muscle cells as also playing an important role in contributing to coronary vascular biomechanics. Indeed, cellular stiffness is an emerging component of vessel biomechanics and there have been previous studies outside of the coronary field that demonstrate this point well. Studies have reported an increase in aortic (macrovascular) VSMC and endothelial cell stiffness in Western-diet-fed animal models, and in many cases, these were associated with increased vessel stiffness (75–78). Very recent data from our laboratory showed that coronary microvascular VSMCs were less stiff in T2DM, in keeping with a consistent decrease in coronary microvascular tissue stiffness (79). Collectively, these data combined with other data in VSMCs from larger arteries suggest that cell stiffness and vascular wall stiffness are linked (80). The mechanisms governing cell stiffness and the influence it ultimately has in regulating CBF have yet to be fully elucidated. The content and organization of cytoskeletal proteins have been a subject of recent investigations (81–86), as have the Rho-associated protein kinase (ROCK) and serum response factor (SRF)/myocardin signaling pathways (87). Conversely, Paul et al. (88) reported that in porcine coronary arteries, the disruption of microtubules by nocodazole elicited significant increases in isometric force, however, little-to-no changes in coronary artery stiffness or unloaded shortening velocity were observed. These data indicate that microtubules do not significantly contribute to coronary vascular smooth muscle mechanical characteristics (88). Although these studies clearly demonstrate a link between cytoskeletal components and cellular stiffness, there is a critical void in the literature linking the role of coronary vascular cell and tissue biomechanics explicitly to the control of CBF. This is a critical void in our knowledge and should be an active area of investigation.
Forces Acting on the Coronary Circulation
The coronary vessels experience force from both the myocardium surrounding the vessel and the blood flowing through it. These forces can be categorized into three principal stresses (circumferential, axial, and radial) and shear stress. Circumferential (or hoop) stress is the force within the vessel wall circumferentially, originating from internal pressure that acts tangentially to increase the circumference of the vessel. Axial stress is the force acting along the longitudinal direction of the vessel also due to internal pressure. Radial stress is the stress that is normal to the inner walls of the vessel and is zero on the outer wall. In the case of thin-walled pressurized vessels, such as the coronary microcirculation, radial stress is insignificant compared with the other two principal stresses. Finally, wall shear stress (WSS) is the drag force along the vessel wall due to blood moving along the endothelial surface. The role of active force in coronary disease progression is currently a popular topic, where it is well known that pathological alterations in localized stresses are often implicated in CAD formation and progression (89–93). For instance, the maintenance of wall stresses in the large conduit coronary arteries is key in preventing atherosclerosis, as localized differences in wall stress are shown to lead to plaque formation (94). There are a number of excellent reviews on the topic that provide an in-depth discussion of the role of both shear stress and radial stress on coronary artery disease and atherosclerosis (95–97). However, very little is known about how forces in the coronary vasculature regulate and maintain CBF.
Computational modeling of the active and passive biomechanics of the coronary vasculature is becoming more and more popular as a means of analyzing the effect of forces on CBF, in part because of the complexities of measuring them in vivo. Waters et al. (98) provide an excellent in-depth review of the current state of theoretical and computational modeling of coronary biomechanics. Although much of the current modeling of the coronary vasculature focuses on the effects on atherosclerosis progression (99–101), there also exists a focus on the relationship between external force application (extravascular compression) and CBF. Models of the coronary conduit arteries reveal the relationship between a vessel’s material properties (passive mechanics) and flow through the vessel, where flow is almost entirely dependent on the stiffness and functionality of the vessel (smooth muscle contraction) (102–104). In the microvasculature, however, external force application seems to play a significant role, likely due to the thin-walled nature of the smaller vessels (105, 106). Recent models point to the impact of myocardial mechanics in regulation of coronary flow, where extravascular compression decreases the radius of embedded vessels and subsequent CBF (101, 107–109). Although there still remains a large gap in modeling passive biomechanics of the microvessels, recent developments in computational modeling of the coronary vasculature have shed light on mechanical regulation of coronary CBF.
THE COMPLEXITIES OF THE INTEGRATION OF STRUCTURAL AND BIOMECHANICAL INFLUENCES OF CBF: A SUMMARY
Over the past five decades, substantial studies have primarily focused on elucidating the functional mechanisms regulating CBF; however, in more recent years there has been a significant amount of evidence that shows the importance of both structural changes and biomechanical alterations in regulating CBF. It is well established that myogenic, biochemical, endothelial, and neural-mediated functions regulate the coronary circulation; however, the integration of these signals and how these individual functional components all work together to regulate coronary perfusion is not well known in large part because studying them in an integrated manner is extremely complicated. The utilization of computational models may facilitate the answers to these relative questions. In the meantime, one possible explanation of these relationships could lie within timing and disease status. For example, functional alterations (VSMC constriction/dilation) within the coronary circulation can occur more quickly than changes in remodeling or biomechanics, as those involve adding/reorganizing cells and extracellular matrix. Therefore, it may be that coronary function dictates acute modulations of CBF, whereas alterations in remodeling and biomechanics influence CBF more chronically while still retaining acute functional regulation, albeit impaired in diseases such as diabetes and aging. These factors are compounded by the fact that the myocardium itself can alter CBF under various pathological conditions (3, 10). Finally, given our previous findings of reduced radius, reduced stiffness, and reduced CBF in the coronary microcirculation of both mouse and pig models of disease (11, 12), it is tempting to speculate that alterations in biomechanical components (e.g., coronary stiffness) serve to compensate to prevent further reductions in CBF in these conditions. Future studies are warranted to more fully elucidate these relationships.
The current plethora of research studies in these various facets point out the complexity of the many mechanisms that are engaged and working together to control CBF. With so many factors influencing CBF, we must take on a new multidisciplinary approach to studying the mechanisms with a particular focus on including raw radius/diameter data in studies to conduct direct comparisons. Those data in the literature are scarce as most functional data are presented as a percent of baseline or preconstricted value, but there are a few examples that indeed show that myogenic responses and remodeling can have huge impacts on diameter and flow (11, 12, 110, 111). For example, Poiseuille’s Law would estimate a ∼98% reduction in CBF due to myogenic responses in mouse coronary arterioles, when compared to passive radius (111). Similarly, passive remodeling radius changes in our own data between normal and diabetic mice and pigs would estimate a 45%–50% reduction in CBF (11, 12), which is in keeping with our actual reported 40%–45% reductions in hyperemic CBF (11). These components have been well studied as individual contributors, and we must now approach our study of them as an integrated unit in normal and disease states, perhaps using computational biology as a vehicle. With this approach, it will become much simpler to elucidate which mechanisms are compensatory versus direct results of the disease state. Given these arguments, future studies should address the interface among structure-function relationships coordinated with the poorly understood influence of coronary biomechanics and myocardial “extravascular compression.”
GRANTS
This work was supported in part by the National Institutes of Health Grants R00 HL116769, R21 EB026518, and S10 OD023438 (to A.J.T.) and The Abigail Wexner Research Institute at Nationwide Children’s Hospital (to A.J.T.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.E.M., B.W.S., and A.J.T. conceived and designed research; P.E.M., B.W.S., and A.J.T. analyzed data; P.E.M., B.W.S., and A.J.T. interpreted results of experiments; P.E.M., B.W.S., and A.J.T. prepared figures; P.E.M., B.W.S., and A.J.T. drafted manuscript; P.E.M., B.W.S., and A.J.T. edited and revised manuscript; P.E.M., B.W.S., and A.J.T. approved final version of manuscript.
REFERENCES
- 1.Muller-Delp JM. The coronary microcirculation in health and disease. ISRN Physiology 2013: 238979, 2013. doi: 10.1155/2013/238979. [DOI] [Google Scholar]
- 2.Muller JM, Davis MJ, Chilian WM. Integrated regulation of pressure and flow in the coronary microcirculation. Cardiovasc Res 32: 668–678, 1996. doi: 10.1016/S0008-6363(96)00111-3. [DOI] [PubMed] [Google Scholar]
- 3.Goodwill AG, Dick GM, Kiel AM, Tune JD. Regulation of coronary blood flow. Compr Physiol 7: 321–382, 2017. doi: 10.1002/cphy.c160016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chilian WM, Eastham CL, Marcus ML. Microvascular distribution of coronary vascular resistance in beating left ventricle. Am J Physiol 251: H779–H788, 1986. doi: 10.1152/ajpheart.1986.251.4.H779. [DOI] [PubMed] [Google Scholar]
- 5.Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med 340: 115–126, 1999. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 6.VanderLaan PA, Reardon CA, Getz GS. Site specificity of atherosclerosis: site-selective responses to atherosclerotic modulators. Arterioscler Thromb Vasc Biol 24: 12–22, 2004. doi: 10.1161/01.ATV.0000105054.43931.f0. [DOI] [PubMed] [Google Scholar]
- 7.Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res 90: 251–262, 2002. doi: 10.1161/res.90.3.251. [DOI] [PubMed] [Google Scholar]
- 8.Liu J, Sukhova GK, Sun JS, Xu WH, Libby P, Shi GP. Lysosomal cysteine proteases in atherosclerosis. Arterioscler Thromb Vasc Biol 24: 1359–1366, 2004. doi: 10.1161/01.ATV.0000134530.27208.41. [DOI] [PubMed] [Google Scholar]
- 9.Gould KL, Lipscomb K. Effects of coronary stenoses on coronary flow reserve and resistance. Am J Cardiol 34: 48–55, 1974. doi: 10.1016/0002-9149(74)90092-7. [DOI] [PubMed] [Google Scholar]
- 10.Duncker DJ, Koller A, Merkus D, Canty JM Jr.. Regulation of coronary blood flow in health and ischemic heart disease. Prog Cardiovasc Dis 57: 409–422, 2015. doi: 10.1016/j.pcad.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katz PS, Trask AJ, Souza-Smith FM, Hutchinson KR, Galantowicz ML, Lord KC, Stewart JA Jr, Cismowski MJ, Varner KJ, Lucchesi PA. Coronary arterioles in type 2 diabetic (db/db) mice undergo a distinct pattern of remodeling associated with decreased vessel stiffness. Basic Res Cardiol 106: 1123–1134, 2011. doi: 10.1007/s00395-011-0201-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trask AJ, Katz PS, Kelly AP, Galantowicz ML, Cismowski MJ, West TA, Neeb ZP, Berwick ZC, Goodwill AG, Alloosh M, Tune JD, Sturek M, Lucchesi PA. Dynamic micro- and macrovascular remodeling in coronary circulation of obese Ossabaw pigs with metabolic syndrome. J Appl Physiol (1985) 113: 1128–1140, 2012. doi: 10.1152/japplphysiol.00604.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanna MA, Taylor CR, Chen B, La HS, Maraj JJ, Kilar CR, Behnke BJ, Delp MD, Muller-Delp JM. Structural remodeling of coronary resistance arteries: effects of age and exercise training. J Appl Physiol (1985) 117: 616–623, 2014. doi: 10.1152/japplphysiol.01296.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spaan J, Kolyva C, van den Wijngaard J, ter Wee R, van Horssen P, Piek J, Siebes M. Coronary structure and perfusion in health and disease. Philos Trans A Math Phys Eng Sci 366: 3137–3153, 2008. doi: 10.1098/rsta.2008.0075. [DOI] [PubMed] [Google Scholar]
- 15.McCallinhart PE, Sunyecz IL, Trask AJ. Coronary microvascular remodeling in type 2 diabetes: synonymous with early aging? Front Physiol 9: 1463, 2018. doi: 10.3389/fphys.2018.01463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Husarek KE, Katz PS, Trask AJ, Galantowicz ML, Cismowski MJ, Lucchesi PA. The angiotensin receptor blocker losartan reduces coronary arteriole remodeling in type 2 diabetic mice. Vasc Pharmacol 76: 28–36, 2016. doi: 10.1016/j.vph.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rocic P. Why is coronary collateral growth impaired in type II diabetes and the metabolic syndrome? Vasc Pharmacol 57: 179–186, 2012. doi: 10.1016/j.vph.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yun J, Rocic P, Pung YF, Belmadani S, Carrao AC, Ohanyan V, Chilian WM. Redox-dependent mechanisms in coronary collateral growth: the “redox window” hypothesis. Antioxid Redox Signal 11: 1961–1974, 2009. doi: 10.1089/ars.2009.2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chilian WM, Penn MS, Pung YF, Dong F, Mayorga M, Ohanyan V, Logan S, Yin L. Coronary collateral growth–back to the future. J Mol Cell Cardiol 52: 905–911, 2012. doi: 10.1016/j.yjmcc.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jamaiyar A, Juguilon C, Dong F, Cumpston D, Enrick M, Chilian WM, Yin L. Cardioprotection during ischemia by coronary collateral growth. Am J Physiol Heart Circ Physiol 316: H1–H9, 2019. doi: 10.1152/ajpheart.00145.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meier P, Schirmer SH, Lansky AJ, Timmis A, Pitt B, Seiler C. The collateral circulation of the heart. BMC Med 11: 143, 2013. doi: 10.1186/1741-7015-11-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stoller M, Seiler C. Pathophysiology of coronary collaterals. Curr Cardiol Rev 10: 38–56, 2014. doi: 10.2174/1573403X113099990005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen H, Kassab GS. Microstructure-based biomechanics of coronary arteries in health and disease. J Biomech 49: 2548–2559, 2016. doi: 10.1016/j.jbiomech.2016.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mackenzie IS, Wilkinson IB, Cockcroft JR. Assessment of arterial stiffness in clinical practice. QJM 95: 67–74, 2002. doi: 10.1093/qjmed/95.2.67. [DOI] [PubMed] [Google Scholar]
- 25.Shirwany NA, Zou MH. Arterial stiffness: a brief review. Acta Pharmacol Sin 31: 1267–1276, 2010. doi: 10.1038/aps.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anghelescu M, Tonniges JR, Calomeni E, Shamhart PE, Agarwal G, Gooch KJ, Trask AJ. Vascular mechanics in decellularized aortas and coronary resistance microvessels in type 2 diabetic db/db mice. Ann Biomed Eng 43: 2760–2770, 2015. doi: 10.1007/s10439-015-1333-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Humphrey JD. Stress, strain, and mechanotransduction in cells. J Biomech Eng 123: 638–641, 2001. doi: 10.1115/1.1406131. [DOI] [PubMed] [Google Scholar]
- 28.Nigam A, Mitchell GF, Lambert J, Tardif JC. Relation between conduit vessel stiffness (assessed by tonometry) and endothelial function (assessed by flow-mediated dilatation) in patients with and without coronary heart disease. Am J Cardiol 92: 395–399, 2003. doi: 10.1016/S0002-9149(03)00656-8. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki E, Kashiwagi A, Nishio Y, Egawa K, Shimizu S, Maegawa H, Haneda M, Yasuda H, Morikawa S, Inubushi T, Kikkawa R. Increased arterial wall stiffness limits flow volume in the lower extremities in type 2 diabetic patients. Diabetes Care 24: 2107–2114, 2001. doi: 10.2337/diacare.24.12.2107. [DOI] [PubMed] [Google Scholar]
- 30.DeLoach SS, Townsend RR. Vascular stiffness: its measurement and significance for epidemiologic and outcome studies. Clin J Am Soc Nephrol 3: 184–192, 2008. doi: 10.2215/CJN.03340807. [DOI] [PubMed] [Google Scholar]
- 31.Izzo JL, Jr, Shykoff BE. Arterial stiffness: clinical relevance, measurement, and treatment. Rev Cardiovasc Med 2: 37–40, 2001. [PubMed] [Google Scholar]
- 32.Benetos A, Waeber B, Izzo J, Mitchell G, Resnick L, Asmar R, Safar M. Influence of age, risk factors, and cardiovascular and renal disease on arterial stiffness: clinical applications. Am J Hypertens 15: 1101–1108, 2002. doi: 10.1016/S0895-7061(02)03029-7. [DOI] [PubMed] [Google Scholar]
- 33.Doonan RJ, Hausvater A, Scallan C, Mikhailidis DP, Pilote L, Daskalopoulou SS. The effect of smoking on arterial stiffness. Hypertens Res 33: 398–410, 2010. doi: 10.1038/hr.2010.25. [DOI] [PubMed] [Google Scholar]
- 34.Liao J, Farmer J. Arterial stiffness as a risk factor for coronary artery disease. Curr Atheroscler Rep 16: 387, 2014. doi: 10.1007/s11883-013-0387-8. [DOI] [PubMed] [Google Scholar]
- 35.Mahmud A, Feely J. Effect of smoking on arterial stiffness and pulse pressure amplification. Hypertension 41: 183–187, 2003. doi: 10.1161/01.HYP.0000047464.66901.60. [DOI] [PubMed] [Google Scholar]
- 36.Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, Asmar R, Reneman RS, Hoeks AP, Breteler MM, Witteman JC. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation 113: 657–663, 2006. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 37.Weber T, Auer J, O’Rourke MF, Kvas E, Lassnig E, Berent R, Eber B. Arterial stiffness, wave reflections, and the risk of coronary artery disease. Circulation 109: 184–189, 2004. doi: 10.1161/01.CIR.0000105767.94169.E3. [DOI] [PubMed] [Google Scholar]
- 38.Cusma-Piccione M, Zito C, Khandheria BK, Pizzino F, Di Bella G, Antonini-Canterin F, Vriz O, Bello VA, Zimbalatti C, La Carrubba S, Oreto G, Carerj S. How arterial stiffness may affect coronary blood flow: a challenging pathophysiological link. J Cardiovasc Med (Hagerstown) 15: 797–802, 2014. doi: 10.2459/JCM.0000000000000185. [DOI] [PubMed] [Google Scholar]
- 39.Fukuda D, Yoshiyama M, Shimada K, Yamashita H, Ehara S, Nakamura Y, Kamimori K, Tanaka A, Kawarabayashi T, Yoshikawa J. Relation between aortic stiffness and coronary flow reserve in patients with coronary artery disease. Heart 92: 759–762, 2005. doi: 10.1136/hrt.2005.067934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leung MC, Meredith IT, Cameron JD. Aortic stiffness affects the coronary blood flow response to percutaneous coronary intervention. Am J Physiol Heart Circ Physiol 290: H624–H630, 2006. doi: 10.1152/ajpheart.00380.2005. [DOI] [PubMed] [Google Scholar]
- 41.Liu J, Wang Y, An H, Liu J, Wei J, Wang H, Wang G. Correlation between arterial stiffness and coronary flow velocity reserve in subjects with pulse wave velocity >1400 cm/s. Clin Exp Hypertens 38: 89–94, 2016. doi: 10.3109/10641963.2015.1060988. [DOI] [PubMed] [Google Scholar]
- 42.Recchia FA, Senzaki H, Saeki A, Byrne BJ, Kass DA. Pulse pressure-related changes in coronary flow in vivo are modulated by nitric oxide and adenosine. Circ Res 79: 849–856, 1996. doi: 10.1161/01.RES.79.4.849. [DOI] [PubMed] [Google Scholar]
- 43.Saeki A, Recchia F, Kass DA. systolic flow augmentation in hearts ejecting into a model of stiff aging vasculature. Influence on myocardial perfusion-demand balance. Circ Res 76: 132–141, 1995. doi: 10.1161/01.RES.76.1.132. [DOI] [PubMed] [Google Scholar]
- 44.Bovendeerd PH, Borsje P, Arts T, van De Vosse FN. Dependence of intramyocardial pressure and coronary flow on ventricular loading and contractility: a model study. Ann Biomed Eng 34: 1833–1845, 2006. doi: 10.1007/s10439-006-9189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krams R, Sipkema P, Zegers J, Westerhof N. Contractility is the main determinant of coronary systolic flow impediment. Am J Physiol 257: H1936–H1944, 1989. doi: 10.1152/ajpheart.1989.257.6.H1936. [DOI] [PubMed] [Google Scholar]
- 46.Spaan JA, Breuls NP, Laird JD. Diastolic-systolic coronary flow differences are caused by intramyocardial pump action in the anesthetized dog. Circ Res 49: 584–593, 1981. doi: 10.1161/01.RES.49.3.584. [DOI] [PubMed] [Google Scholar]
- 47.Gregg DE, Khouri EM, Rayford CR. Systemic and coronary energetics in the resting unanesthetized dog. Circ Res 16: 102–113, 1965. doi: 10.1161/01.RES.16.2.102. [DOI] [PubMed] [Google Scholar]
- 48.Gregg DE, Sabiston DC Jr.. Effect of cardiac contraction on coronary blood flow. Circulation 15: 14–20, 1957. doi: 10.1161/01.CIR.15.1.14. [DOI] [PubMed] [Google Scholar]
- 49.Lowensohn HS, Khouri EM, Gregg DE, Pyle RL, Patterson RE. Phasic right coronary artery blood flow in conscious dogs with normal and elevated right ventricular pressures. Circ Res 39: 760–766, 1976. doi: 10.1161/01.RES.39.6.760. [DOI] [PubMed] [Google Scholar]
- 50.Downey JM, Kirk ES. Inhibition of coronary blood flow by a vascular waterfall mechanism. Circ Res 36: 753–760, 1975. doi: 10.1161/01.RES.36.6.753. [DOI] [PubMed] [Google Scholar]
- 51.Gosselin RE, Kaplow SM. Venous waterfalls in coronary circulation. J Theor Biol 149: 265–279, 1991. doi: 10.1016/S0022-5193(05)80281-4. [DOI] [PubMed] [Google Scholar]
- 52.Maas JJ, de Wilde RB, Aarts LP, Pinsky MR, Jansen JR. Determination of vascular waterfall phenomenon by bedside measurement of mean systemic filling pressure and critical closing pressure in the intensive care unit. Anesth Analg 114: 803–810, 2012. doi: 10.1213/ANE.0b013e318247fa44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Uhlig PN, Baer RW, Vlahakes GJ, Hanley FL, Messina LM, Hoffman JI. Arterial and venous coronary pressure-flow relations in anesthetized dogs. Evidence for a vascular waterfall in epicardial coronary veins. Circ Res 55: 238–248, 1984. doi: 10.1161/01.RES.55.2.238. [DOI] [PubMed] [Google Scholar]
- 54.Badeer HS, Hicks JW. Hemodynamics of vascular 'waterfall': is the analogy justified? Respir Physiol 87: 205–217, 1992. doi: 10.1016/0034-5687(92)90060-A. [DOI] [PubMed] [Google Scholar]
- 55.Westerhof N, Sipkema P, Van Huis GA. Coronary pressure-flow relations and the vascular waterfall. Cardiovasc Res 17: 162–169, 1983. doi: 10.1093/cvr/17.3.162. [DOI] [PubMed] [Google Scholar]
- 56.Mynard JP, Penny DJ, Smolich JJ. Major influence of a 'smoke and mirrors' effect caused by wave reflection on early diastolic coronary arterial wave intensity. J Physiol 596: 993–1017, 2018. doi: 10.1113/JP274710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zamir M. Mechanics of blood supply to the heart: wave reflection effects in a right coronary artery. Proc Biol Sci 265: 439–444, 1998. doi: 10.1098/rspb.1998.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lekakis JP, Ikonomidis I, Protogerou AD, Papaioannou TG, Stamatelopoulos K, Papamichael CM, Mavrikakis ME. Arterial wave reflection is associated with severity of extracoronary atherosclerosis in patients with coronary artery disease. Eur J Cardiovasc Prev Rehabil 13: 236–242, 2006. doi: 10.1097/01.hjr.0000198446.18989.4f. [DOI] [PubMed] [Google Scholar]
- 59.Canty JM, Jr. Coronary pressure-function and steady-state pressure-flow relations during autoregulation in the unanesthetized dog. Circ Res 63: 821–836, 1988. doi: 10.1161/01.RES.63.4.821. [DOI] [PubMed] [Google Scholar]
- 60.Chilian WM, Marcus ML. Phasic coronary blood flow velocity in intramural and epicardial coronary arteries. Circ Res 50: 775–781, 1982. doi: 10.1161/01.RES.50.6.775. [DOI] [PubMed] [Google Scholar]
- 61.Toyota E, Ogasawara Y, Hiramatsu O, Tachibana H, Kajiya F, Yamamori S, Chilian WM. Dynamics of flow velocities in endocardial and epicardial coronary arterioles. Am J Physiol Heart Cir Physiol 288: H1598–H1603, 2005. doi: 10.1152/ajpheart.01103.2003. [DOI] [PubMed] [Google Scholar]
- 62.Cobb FR, Bache RJ, Greenfield JC Jr.. Regional myocardial blood flow in awake dogs. J Clin Invest 53: 1618–1625, 1974. doi: 10.1172/JCI107712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weintraub WS, Hattori S, Agarwal J, Bodenheimer MM, Banka VS, Helfant RH. Variable effect of nifedipine on myocardial blood flow at three grades of coronary occlusion in the dog. Circ Res 48: 937–942, 1981. doi: 10.1161/01.RES.48.6.937. [DOI] [PubMed] [Google Scholar]
- 64.Weintraub WS, Hattori S, Agarwal JB, Bodenheimer MM, Banka VS, Helfant RH. The relationship between myocardial blood flow and contraction by myocardial layer in the canine left ventricle during ischemia. Circ Res 48: 430–438, 1981. doi: 10.1161/01.RES.48.3.430. [DOI] [PubMed] [Google Scholar]
- 65.Ramanathan T, Skinner H. Coronary blood flow. Cont Educ Anaesth Crit Care Pain 5: 61–64, 2005. doi: 10.1093/bjaceaccp/mki012. [DOI] [Google Scholar]
- 66.Douglas JE, Greenfield JC Jr.. Epicardial coronary artery compliance in the dog. Circ Res 27: 921–929, 1970. doi: 10.1161/01.RES.27.6.921. [DOI] [PubMed] [Google Scholar]
- 67.Reneman RS, Arts T. Dynamic capacitance of epicardial coronary arteries in vivo. J Biomech Eng 107: 29–33, 1985. doi: 10.1115/1.3138515. [DOI] [PubMed] [Google Scholar]
- 68.Sun Y, Gewirtz H. Characterization of the coronary vascular capacitance, resistance, and flow in endocardium and epicardium based on a nonlinear dynamic analog model. IEEE Trans Biomed Eng 34: 817–825, 1987. doi: 10.1109/TBME.1987.325924. [DOI] [PubMed] [Google Scholar]
- 69.Goto M, Flynn AE, Doucette JW, Jansen CM, Stork MM, Coggins DL, Muehrcke DD, Husseini WK, Hoffman JI. Cardiac contraction affects deep myocardial vessels predominantly. Am J Physiol 261: H1417–H1429, 1991. doi: 10.1152/ajpheart.1991.261.5.H1417. [DOI] [PubMed] [Google Scholar]
- 70.Spaan JA. Coronary diastolic pressure-flow relation and zero flow pressure explained on the basis of intramyocardial compliance. Circ Res 56: 293–309, 1985. doi: 10.1161/01.RES.56.3.293. [DOI] [PubMed] [Google Scholar]
- 71.Chilian WM. Microvascular pressures and resistances in the left ventricular subepicardium and subendocardium. Circ Res 69: 561–570, 1991. doi: 10.1161/01.RES.69.3.561. [DOI] [PubMed] [Google Scholar]
- 72.Wagenseil JE, Mecham RP. Vascular extracellular matrix and arterial mechanics. Physiological reviews 89: 957–989, 2009. doi: 10.1152/physrev.00041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wagenseil JE, Ciliberto CH, Knutsen RH, Levy MA, Kovacs A, Mecham RP. The importance of elastin to aortic development in mice. Am J Physiol Heart Circ Physiol 299: H257–H264, 2010. doi: 10.1152/ajpheart.00194.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yanagisawa H, Wagenseil J. Elastic fibers and biomechanics of the aorta: insights from mouse studies. Matrix Biol 85–86: 160–172, 2020. doi: 10.1016/j.matbio.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Aroor AR, Das NA, Carpenter AJ, Habibi J, Jia G, Ramirez-Perez FI, Martinez-Lemus L, Manrique-Acevedo CM, Hayden MR, Duta C, Nistala R, Mayoux E, Padilla J, Chandrasekar B, DeMarco VG. Glycemic control by the SGLT2 inhibitor empagliflozin decreases aortic stiffness, renal resistivity index and kidney injury. Cardiovasc Diabetol 17: 108, 2018. doi: 10.1186/s12933-018-0750-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.DeMarco VG, Habibi J, Jia G, Aroor AR, Ramirez-Perez FI, Martinez-Lemus LA, Bender SB, Garro M, Hayden MR, Sun Z, Meininger GA, Manrique C, Whaley-Connell A, Sowers JR. Low-dose mineralocorticoid receptor blockade prevents western diet-induced arterial stiffening in female mice. Hypertension 66: 99–107, 2015. doi: 10.1161/HYPERTENSIONAHA.115.05674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jia G, Habibi J, Aroor AR, Martinez-Lemus LA, DeMarco VG, Ramirez-Perez FI, Sun Z, Hayden MR, Meininger GA, Mueller KB, Jaffe IZ, Sowers JR. Endothelial mineralocorticoid receptor mediates diet-induced aortic stiffness in females. Circ Res 118: 935–943, 2016. doi: 10.1161/CIRCRESAHA.115.308269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Manrique C, Habibi J, Aroor AR, Sowers JR, Jia G, Hayden MR, Garro M, Martinez-Lemus LA, Ramirez-Perez FI, Klein T, Meininger GA, DeMarco VG. Dipeptidyl peptidase-4 inhibition with linagliptin prevents western diet-induced vascular abnormalities in female mice. Cardiovasc Diabetol 15: 94, 2016. doi: 10.1186/s12933-016-0414-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McCallinhart PE, Cho Y, Sun Z, Ghadiali S, Meininger GA, Trask AJ. Reduced stiffness and augmented traction force in type 2 diabetic coronary microvascular smooth muscle. Am J Physiol Heart Circ Physiol 318: H1410–H1419, 2020. doi: 10.1152/ajpheart.00542.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Meininger GA. The central importance of the cytoskeleton for increased cell stiffness in cardiovascular disease. Focus on “Diabetes increases stiffness of live cardiomyocytes measured by atomic force microscopy nanoindentation”. Am J Physiol Cell Physiol 307: C908–C909, 2014. doi: 10.1152/ajpcell.00279.2014. [DOI] [PubMed] [Google Scholar]
- 81.Hong Z, Sun Z, Li M, Li Z, Bunyak F, Ersoy I, Trzeciakowski JP, Staiculescu MC, Jin M, Martinez-Lemus L, Hill MA, Palaniappan K, Meininger GA. Vasoactive agonists exert dynamic and coordinated effects on vascular smooth muscle cell elasticity, cytoskeletal remodelling and adhesion. J Physiol 592: 1249–1266, 2014. doi: 10.1113/jphysiol.2013.264929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hong Z, Sun Z, Li Z, Mesquitta WT, Trzeciakowski JP, Meininger GA. Coordination of fibronectin adhesion with contraction and relaxation in microvascular smooth muscle. Cardiovasc Res 96: 73–80, 2012. doi: 10.1093/cvr/cvs239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jia G, Habibi J, DeMarco VG, Martinez-Lemus LA, Ma L, Whaley-Connell AT, Aroor AR, Domeier TL, Zhu Y, Meininger GA, Mueller KB, Jaffe IZ, Sowers JR. Endothelial mineralocorticoid receptor deletion prevents diet-induced cardiac diastolic dysfunction in females. Hypertension 66: 1159–1167, 2015. doi: 10.1161/HYPERTENSIONAHA.115.06015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lacolley P, Regnault V, Avolio AP. Smooth muscle cell and arterial aging: basic and clinical aspects. Cardiovasc Res 114: 513–528, 2018. doi: 10.1093/cvr/cvy009. [DOI] [PubMed] [Google Scholar]
- 85.Saphirstein RJ, Gao YZ, Lin QQ, Morgan KG. Cortical actin regulation modulates vascular contractility and compliance in veins. J Physiol 593: 3929–3941, 2015. doi: 10.1113/JP270845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sehgel NL, Zhu Y, Sun Z, Trzeciakowski JP, Hong Z, Hunter WC, Vatner DE, Meininger GA, Vatner SF. Increased vascular smooth muscle cell stiffness: a novel mechanism for aortic stiffness in hypertension. Am J Physiol Heart Circ Physiol 305: H1281–H1287, 2013. doi: 10.1152/ajpheart.00232.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhou N, Lee JJ, Stoll S, Ma B, Costa KD, Qiu H. Rho kinase regulates aortic vascular smooth muscle cell stiffness via actin/SRF/myocardin in hypertension. Cell Physiol Biochem 44: 701–715, 2017. doi: 10.1159/000485284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Paul RJ, Bowman PS, Kolodney MS. Effects of microtubule disruption on force, velocity, stiffness and [Ca(2+)](i) in porcine coronary arteries. Am J Physiology Heart Circ Physiol 279: H2493–H2501, 2000. doi: 10.1152/ajpheart.2000.279.5.H2493. [DOI] [PubMed] [Google Scholar]
- 89.Caro CG, Fitz-Gerald JM, Schroter RC. Arterial wall shear and distribution of early atheroma in man. Nature 223: 1159–1160, 1969. doi: 10.1038/2231159a0. [DOI] [PubMed] [Google Scholar]
- 90.Corban MT, Eshtehardi P, Suo J, McDaniel MC, Timmins LH, Rassoul-Arzrumly E, Maynard C, Mekonnen G, King S 3rd, Quyyumi AA, Giddens DP, Samady H. Combination of plaque burden, wall shear stress, and plaque phenotype has incremental value for prediction of coronary atherosclerotic plaque progression and vulnerability. Atherosclerosis 232: 271–276, 2014. doi: 10.1016/j.atherosclerosis.2013.11.049. [DOI] [PubMed] [Google Scholar]
- 91.Gimbrone MA Jr, Topper JN, Nagel T, Anderson KR, Garcia-Cardeña G. Endothelial dysfunction, hemodynamic forces, and atherogenesis. Ann N Y Acad Sci 902: 230–239, 2000. doi: 10.1111/j.1749-6632.2000.tb06318.x. [DOI] [PubMed] [Google Scholar]
- 92.Karimi A, Navidbakhsh M, Shojaei A, Faghihi S. Measurement of the uniaxial mechanical properties of healthy and atherosclerotic human coronary arteries. Mater Sci Eng C Mater Biol Appl 33: 2550–2554, 2013. doi: 10.1016/j.msec.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 93.Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA 282: 2035–2042, 1999. doi: 10.1001/jama.282.21.2035. [DOI] [PubMed] [Google Scholar]
- 94.Chatzizisis YS, Giannoglou GD. Coronary hemodynamics and atherosclerotic wall stiffness: a vicious cycle. Med Hypotheses 69: 349–355, 2007. doi: 10.1016/j.mehy.2006.11.053. [DOI] [PubMed] [Google Scholar]
- 95.Brown AJ, Teng Z, Evans PC, Gillard JH, Samady H, Bennett MR. Role of biomechanical forces in the natural history of coronary atherosclerosis. Nat Rev Cardiol 13: 210–220, 2016. doi: 10.1038/nrcardio.2015.203. [DOI] [PubMed] [Google Scholar]
- 96.John LC. Biomechanics of coronary artery and bypass graft disease: potential new approaches. Ann Thorac Surg 87: 331–338, 2009. doi: 10.1016/j.athoracsur.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 97.Thondapu V, Bourantas CV, Foin N, Jang IK, Serruys PW, Barlis P. Biomechanical stress in coronary atherosclerosis: emerging insights from computational modelling. Eur Heart J 38: 81–92, 2017. doi: 10.1093/eurheartj/ehv689. [DOI] [PubMed] [Google Scholar]
- 98.Waters SL, Alastruey J, Beard DA, Bovendeerd PH, Davies PF, Jayaraman G, Jensen OE, Lee J, Parker KH, Popel AS, Secomb TW, Siebes M, Sherwin SJ, Shipley RJ, Smith NP, van de Vosse FN. Theoretical models for coronary vascular biomechanics: progress & challenges. Prog Biophys Mol Biol 104: 49–76, 2011. doi: 10.1016/j.pbiomolbio.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gholipour A, Ghayesh MH, Zander A. Nonlinear biomechanics of bifurcated atherosclerotic coronary arteries. Int J Eng Sci 133: 60–83, 2018. doi: 10.1016/j.ijengsci.2018.08.003. [DOI] [Google Scholar]
- 100.Gholipour A, Ghayesh MH, Zander A, Mahajan R. Three-dimensional biomechanics of coronary arteries. Int J Eng Sci 130: 93–114, 2018. doi: 10.1016/j.ijengsci.2018.03.002. [DOI] [Google Scholar]
- 101.Zhang W, Herrera C, Atluri SN, Kassab GS. Effect of surrounding tissue on vessel fluid and solid mechanics. J Biomech Eng 126: 760–769, 2004. doi: 10.1115/1.1824128. [DOI] [PubMed] [Google Scholar]
- 102.Holzapfel GA, Gasser TC, Ogden RW. A new constitutive framework for arterial wall mechanics and a comparative study of material models. J Elasticity 61: 1–48, 2000. doi: 10.1023/A:1010835316564. [DOI] [Google Scholar]
- 103.Humphrey JD. Cardiovascular Solid Mechanics: Cells, Tissues, and Organs. New York: Springer, 2002, p. 758. [Google Scholar]
- 104.Raghavan ML, Vorp DA. Toward a biomechanical tool to evaluate rupture potential of abdominal aortic aneurysm: identification of a finite strain constitutive model and evaluation of its applicability. J Biomechan 33: 475–482, 2000. doi: 10.1016/S0021-9290(99)00201-8. [DOI] [PubMed] [Google Scholar]
- 105.Goto M, VanBavel E, Giezeman MJ, Spaan JA. Vasodilatory effect of pulsatile pressure on coronary resistance vessels. Circ Res 79: 1039–1045, 1996. doi: 10.1161/01.RES.79.5.1039. [DOI] [PubMed] [Google Scholar]
- 106.Sorop O, Spaan JA, Sweeney TE, VanBavel E. Effect of steady versus oscillating flow on porcine coronary arterioles: involvement of NO and superoxide anion. Circ Res 92: 1344–1351, 2003. doi: 10.1161/01.RES.0000078604.47063.2B. [DOI] [PubMed] [Google Scholar]
- 107.Choy JS, Kassab GS. Wall thickness of coronary vessels varies transmurally in the LV but not the RV: implications for local stress distribution. Am J Physiol Heart Circ Physiol 297: H750–H758, 2009. doi: 10.1152/ajpheart.01136.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Smith NP. A computational study of the interaction between coronary blood flow and myocardial mechanics. Physiol Meas 25: 863–877, 2004. doi: 10.1088/0967-3334/25/4/007. [DOI] [PubMed] [Google Scholar]
- 109.Smith NP, Kassab GS. Analysis of coronary blood flow interaction with myocardial mechanics based on anatomical models. Philos T R Soc A 359: 1251–1262, 2001. doi: 10.1098/rsta.2001.0829. [DOI] [Google Scholar]
- 110.Frobert O, Mikkelsen EO, Bagger JP. The influence of transmural pressure and longitudinal stretch on K+- and Ca2+-induced coronary artery constriction. Acta Physiol Scand 165: 379–385, 1999. doi: 10.1046/j.1365-201x.1999.00519.x. [DOI] [PubMed] [Google Scholar]
- 111.Lui AH, McManus BM, Laher I. Endothelial and myogenic regulation of coronary artery tone in the mouse. Eur J Pharmacol 410: 25–31, 2000. doi: 10.1016/S0014-2999(00)00868-2. [DOI] [PubMed] [Google Scholar]