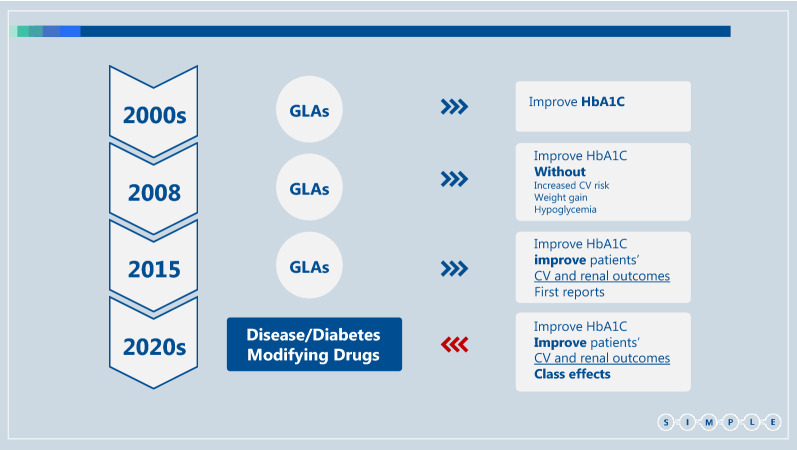
Fig. 1.
Changes in the treatment of patients with type 2 diabetes over the last two decades. GLA Glucose Lowering Agents, CV Cardiovascular, FDA Food and Drugs Administration, DPP4i Dipeptidyl peptidase-4 inhibitors, SGLT2i Sodium glucose co-transporter type 2 inhibitor, GLP-1 RA Glucagon-like peptide-1 receptor agonists, DMD Disease/Diabetes modifying drug, MACE major adverse cardiovascular events. Until the early 2000s treatment of type 2 diabetes focused on glucose control. The main GLAs in use were insulin, metformin and sulfonylureas. In 2008, the FDA started to require pharmaceutical companies to confirm the CV safety of their newly developed GLAs, compared with placebo or other GLA, in patients with high CV risk [82]. Members of the DPP4i and thiazolidinediones classes were found to have CV non-inferiority, without CV superiority, in MACE-based outcomes [55, 83–86]. Starting in 2015, several members of the SGLT2i and GLP-1 RA classes were found to exert cardiorenal protection [10–13, 15–17, 48, 56], defining them as disease/diabetes modifying drug (DMDs)