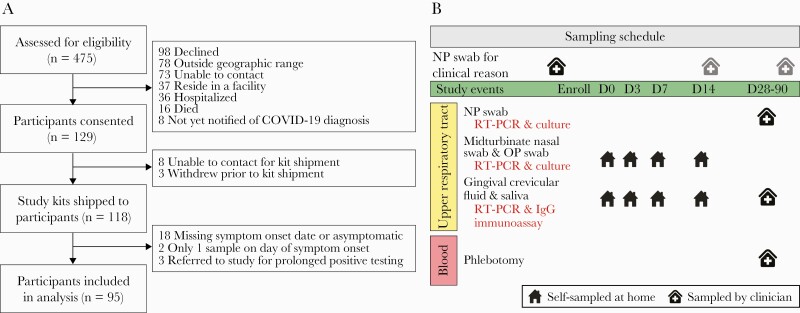
Figure 1.
CONSORT flow diagram and sampling schedule. A, A convenience sample of 475 adults with recent positive SARS-CoV-2 RT-PCR tests from an outpatient testing site of the Johns Hopkins Health System was assessed for eligibility between April 21, 2020, and July 23, 2020. Preference was given to participants 40 years of age and older. Study kits were sent to 118 participants, and data and samples from 95 participants were included in the analyses presented here. B, Study sampling schedule. Clinical RT-PCR results from the medical record are included in these analyses. Samples on study days 0–14 were self-collected at home with telephone or video guidance by trained study staff. Participants presented to a research clinic for collection of samples a median (range) of 45 (27–88) days after study day 0. Abbreviations: RT-PCR, reverse transcription polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.