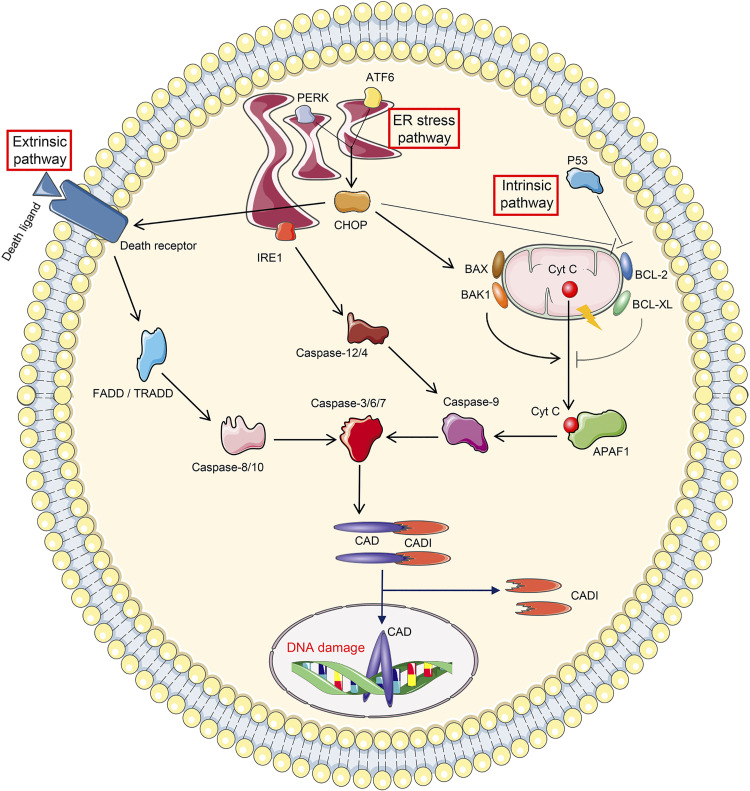
Figure 3.
Cellular events leading to apoptosis. The initiation of apoptosis includes three pathways. Extrinsic pathway apoptosis is initiated by the binding between death receptor and death ligand, followed by sequential activation of Fas-associated death domain (FADD)/tumor necrosis factor receptor-associated death domain (TRADD), caspase-8/10, and caspase-3/6/7. Intrinsic pathway apoptosis is triggered by the mitochondrial leakage of cytochrome c (Cyt C), which binds to apoptotic protease activating factor 1 (APAF1) in the cytoplasm. APAF1 then leads to consequential activation of caspase-9 and caspase-3/6/7. The leakage of Cyt C is strictly controlled by proapoptotic proteins (BAX, BAK1, and P53) and antiapoptotic proteins (BCL-2 and BCL-XL). Endoplasmic reticulum (ER) stress pathway-related apoptosis is mediated by ER stress sensors. Inositol-requiring protein 1 (IRE1) promotes apoptosis by activating successively caspase-12/4, caspase-9, and caspase-3/6/7. Protein kinase R-like ER kinase (PERK) and activating transcription factor 6 (ATF6) drive apoptosis through C/EBP homologous protein (CHOP), which potentiates death receptor activation and mitochondrial permeability. Apoptosis is executed by caspase-3/6/7, among which caspase-3 cleaves inhibitor of caspase-activated DNase (ICAD) to revitalize caspase-activated DNase (CAD), followed by DNA damage.