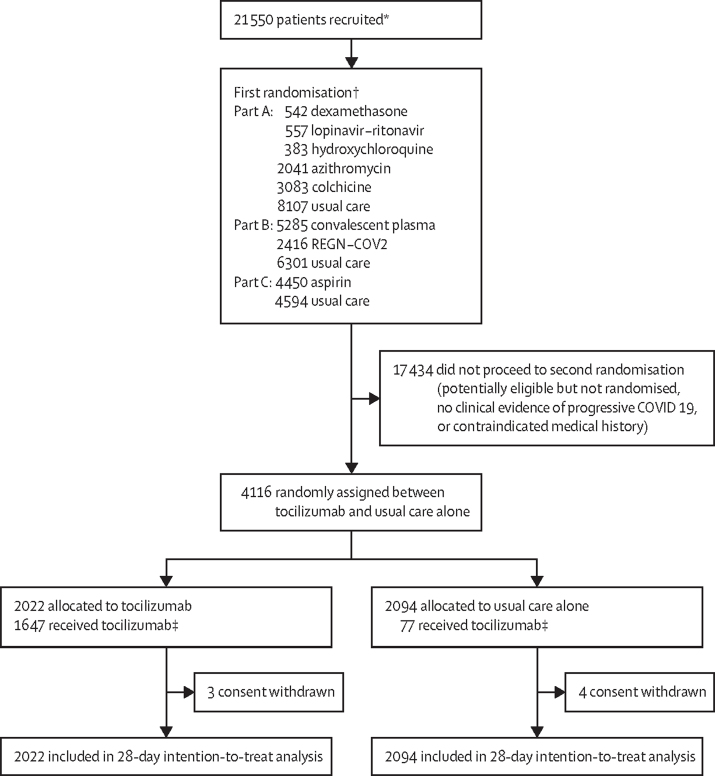
Figure 1.
Trial profile
REGN-COV2=a combination of two monoclonal antibodies directed against SARS-CoV-2 spike protein. *Number of adult patients recruited at a site activated for the tocilizumab comparison. †The first randomisation comprised up to three factorial elements such that an eligible patient could be entered into between one and three randomised comparisons, depending on the then current protocol, the patient's suitability for particular treatments, and the availability of the treatment at the site. Median time between first and second randomisation was 0·3 h (IQR 0·1−25·3). ‡1964 (97%) of 2022 patients of those allocated to tocilizumab and 2049 (98%) of 2094 of those allocated to usual care had a completed follow-up form at time of analysis.