Figure 1.
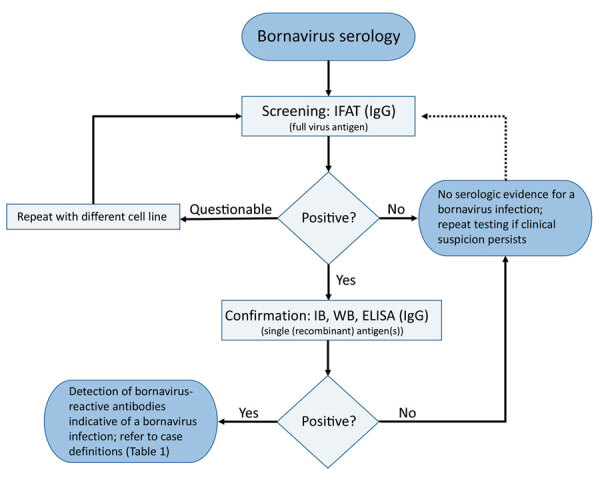
Serologic testing scheme for human bornavirus encephalitis, Germany, 2018–2020. Scheme was based on serologic screening and confirmatory assays and in conjunction with a case definition for variegated squirrel bornavirus 1 (VSBV-1) and Borna disease virus 1 (BoDV-1) encephalitis (Table 1) was diagnosed. Screening of serum samples and cerebrospinal fluid for bornavirus-reactive IgG was conducted by using an indirect immunofluorescence antibody test. A persistently BoDV-1–infected cell line was used with uninfected cells of the same cell line as controls (Vero cells or Crandell-Rees feline kidney cells). For confirmation of a positive IFAT screening result, a line blot with recombinant VSBV-1 and BoDV-1 phosphoprotein proteins was used in our study, but alternative assays, such as WB or ELISA with recombinant antigen(s) or antigen(s) derived from infected cells, might also be appropriate after sufficient validation. Adequate control serum samples from confirmed human VSBV-1 and BoDV-1 encephalitis cases and a pooled serum of 20 healthy blood donors were used for the IFAT and the line blot. IFAT, indirect immunofluorescence antibody test; IB, immunoblot; WB, Western blotting.
