Abstract
Aims
Some studies have reported changes in glycemic control of patients with diabetes mellitus under lockdown. However, no previous study examined the impact of the pandemic on glycemic control in patients with diabetes in countries that did not introduce a lockdown such as Japan. This study aimed to assess changes in glycemic control during the pandemic in patients with type 2 diabetes treated at a Japanese clinic.
Methods
We conducted a historical cohort study, using electronic medical records of patients with type 2 diabetes who visited our clinic between January 2019 and August 2020. Differences in HbA1c values before and after the outbreak of COVID-19 were the primary outcome, examined using the linear mixed model.
Results
HbA1c values significantly increased from 7.45% to 7.53% after the state of emergency was introduced (n = 1,009). Furthermore, a deterioration in HbA1c values was observed in particular among women, patients aged ≥ 65 years, those with body mass index of ≥ 25 kg/m2, and those that were not using insulin.
Conclusions
Glycemic control deteriorated in patients with type 2 diabetes during the pandemic even in a country without a national lockdown.
Keywords: Type 2 diabetes, COVID-19 pandemic, Lockdown, Linear mixed model
1. Introduction
The coronavirus disease 2019 (COVID-19) was first identified in Wuhan, China, in late 2019, before spreading worldwide [1]. The World Health Organization declared the COVID-19 outbreak a pandemic on March 11, 2020 [2]. Subsequently, many countries introduced lockdowns and other types of restrictions to contain the spread of infection. In Japan, the first case of COVID-19 was identified on January 14, 2020; the number of confirmed cases increased rapidly thereafter, and on April 7, 2020, the Japanese government declared a state of emergency, calling on the citizens to remain home and refrain from non-essential outings. This approach was in contrast to that of many other countries, which opted for national lockdowns.
Preliminary research has shown that in countries that introduced lockdowns, patients with diabetes mellitus were subject to treatment delays, and discontinuation of care, service, and medicine supply. In fact, the World Health Organization reported that diabetes treatment was partially or completely disrupted in 49% of 155 countries surveyed in May 2020 [3]. Meanwhile, in Japan, diabetes treatment continued as usual, without evidence of treatment delay or discontinuation. Nevertheless, the state of emergency has affected the nation’s habits, introducing social distancing, the requirement to wear a mask in public, and remote working. Such large-scale behavioral changes may have affected glycemic control among patients with diabetes mellitus even in the absence of a lockdown.
Maintaining good glycemic control during the COVID-19 pandemic is paramount to patients with diabetes mellitus. The deterioration of glycemic control may lead to chronic complications of diabetes mellitus (such as macro- and micro-vascular disease) and increase the risk of infection and associated mortality. In fact, patients with diabetes mellitus have been shown to be at increased risk of morbidity and mortality during the pandemic [4]. Moreover, COVID-19 patients with uncontrolled type 2 diabetes have markedly higher mortality rates than do those with good glycemic control [5].
Previous studies have examined the impact of the COVID-19 pandemic on glycemic control among patients with diabetes mellitus; however, the findings have been inconsistent. For example, a study from Spain reported improved glycemic control in 307 patients with type 1 diabetes after 8 weeks of lockdown compared to before lockdown. These patients were administered multiple daily insulin injections or insulin pump therapy, using continuous glucose monitoring (CGM). This improvement in glycemic control may be due to improved disease self-management under lockdown, made possible by decreased workload, improved diet, and more time for diabetes management [6]. Similar findings were reported in the Netherlands [7], France [8], Italy [9], [10], Greece [11] and by a separate group in Spain [12]. However, these studies included only patients with type 1 diabetes that were using CGM; these patients may have been better placed to manage their disease than were their counterparts, as they could easily monitor their blood glucose levels. However, a study in the UK has shown that glycemic control worsened in patients with type 1 diabetes that were not using CGM [13]. This finding may be accounted for by the restricted availability of insulin or glucostrips under lockdown.
Meanwhile, reports on glycemic control in patients with type 2 diabetes during the COVID-19 pandemic were mixed. A recent study from India reported that glycemic control worsened in 143 patients with type 2 diabetes after 3 weeks of lockdown [14]. This finding may have been due to psychological stress, and difficulty in obtaining medication and medical advice under lockdown. Similar findings were reported in China [15], Korea [16] and by another group in India [17]. In contrast, a separate study from India [18] and one from Greece [19] reported that glycemic control improved; nevertheless, it was reported as unchanged in studies from Italy [20], [21] and Turkey [22]. In addition, a French study has shown that glycemia was less well controlled in patients with gestational diabetes mellitus [23], likely due to reduced physical activity, modified dietary habits, and anxiety experienced under lockdown. Overall, these findings suggest that the impact of the COVID-19 pandemic on glycemic control differs depending on the type of diabetes mellitus and the region of the world.
However, most previous studies were performed in countries that did impose lockdowns, and no previous study has examined the impact of the pandemic on glycemic control of patients with diabetes in a country that did not impose a lockdown. The present study aimed to evaluate the changes in glycemic control associated with the COVID-19 pandemic in Japanese patients with type 2 diabetes.
2. Materials and methods
We performed a historical cohort study that involved reviewing electronic medical records of patients with diabetes mellitus, treated at the Tohoku Medical and Pharmaceutical University Hospital in Sendai, which is a central city in northeast Japan. We included all patients with diabetes mellitus who visited our clinic from January 1, 2019, to August 31, 2020. We excluded patients with type 1 or another type of diabetes, including pancreatic, hepatic, or gestational diabetes, and secondary diabetes from endocrine disease. Only patients with type 2 diabetes were included. Further, we excluded patients with a history of hospitalization during the observation period and those that had accumulated < 180 days of clinical data, starting from their first visit to our clinic (Fig. 1 ). On April 10, 2020, Japan’s Ministry of Health, Labor, and Welfare has granted timely and special permission for medical care using telephones and other remote communication modes during the COVID-19 pandemic. However, only 30 patients from our clinic received medical care via the telephone or other remote communication modes. The remaining patients received regular face-to-face medical care. Thus, almost all patients included in the present study received diabetes care as usual. Baseline characteristics of interest were age, sex, HbA1c values, body mass index (BMI), and insulin use. We examined HbA1c and BMI values before and after the declaration of the state of emergency, assessing the impact of the pandemic on these parameters with the linear mixed model. In the analysis, HbA1c values were considered a random effect; meanwhile, sex, age, BMI, insulin use, and month and year of measurement were considered fixed effects. Similarly, in BMI analysis, baseline BMI values were considered a random effect; meanwhile, sex, age, HbA1c values, insulin use, and month and year of measurement were considered fixed effects. As HbA1c values tend to exhibit seasonal variation in Japan [24], the month of HbA1c measurement was added to the fixed effects. In addition, we performed stratified analyses, based on baseline clinical parameters, including age (<65 years, ≥ 65 years), BMI (<25 kg/m2, ≥ 25 kg/m2), HbA1c values (<7.0%, ≥ 7.0%), and insulin use vs. no insulin use. Results were expressed as mean ± standard deviation or mean and 95% confidence interval. P-values of < 0.05 were considered indicative of a statistically significant finding. Analyses were performed with SAS version 9.4 (SAS Institute, Cary, NC, USA). This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethical Committee of the Tohoku Pharmaceutical University Hospital (2020-2-214). As this was an observational study based on previously collected clinical data, the study was exempt from the informed consent requirement. Instead, relevant information regarding the study was made available to the public and opt-out opportunities were provided.
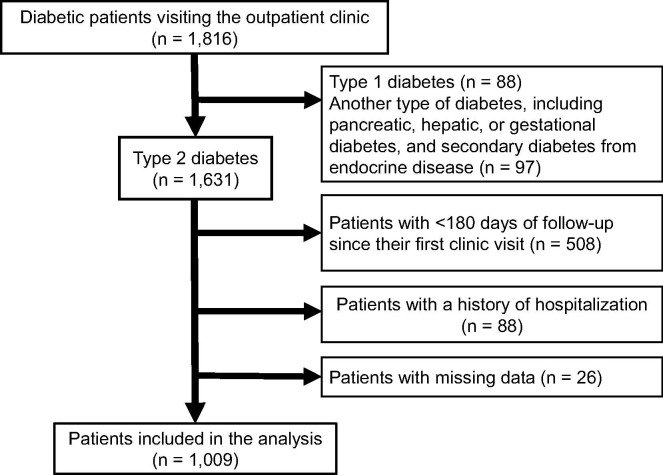
Fig. 1.
Inclusion and exclusion flow This study included 1816 patients with diabetes mellitus who visited our clinic between January 1, 2019, and August 31, 2020. Finally, 1009 patients with type 2 diabetes were included in further analysis.
3. Results
This study included 1,816 patients with diabetes mellitus who visited our clinic between January 1, 2019, and August 31, 2020. We excluded 88 and 97 patients with type 1 or another type of diabetes, respectively. A total of 1,631 patients with type 2 diabetes were included after initial screening; subsequently, we excluded 88, 508, and 26 patients with a history of hospitalization during the observation period, < 180 days of clinical data, and missing clinical data, respectively. Finally, 1,009 patients with type 2 diabetes were included in further analysis (Fig. 1). Baseline characteristics of the selected patients are presented in Table 1 . The proportion of men was 65.1%. The age, HbA1c and BMI values were 64.0 ± 13.6 years, 7.8 ± 1.5% (61.7 ± 11.9 mmol/mol), and 26.4 ± 5.1 kg/m2, respectively. A total of 315 patients were treated with insulin and the daily insulin dose was 27.1 ± 18.3 units. The regimens of insulin treatment were 38.9% for basal-bolus insulin, 19.1% for basal insulin only, 13.0% for bolus insulin only, and 29.0% for premixed insulin. Further, the administration status of oral anti-diabetic drugs and GLP-1 receptor agonist are shown in Table 1. Additionally, in Japan, self-monitoring of blood glucose (SMBG) is currently reimbursed for patients with diabetes receiving insulin injections. In our study, 91.4% of the patients who were treated with insulin performed SMBG, and they measured their blood glucose level 39.9 ± 16.3 times / month. In contrast, patients who were not treated with insulin did not perform SMBG.
Table 1.
Baseline characteristics of study patients with type 2 diabetes.
| Characteristics | Data (n = 1,009) |
|---|---|
| Age (years) | 64.0 ± 13.6 |
| Male, n (%) | 657 (65.1) |
| HbA1c (%) | 7.8 ± 1.5 |
| HbA1c (mmol / mol) | 61.7 ± 11.9 |
| BMI (kg / m2) | 26.4 ± 5.1 |
| Insulin use, n (%) | 315 (31.2) |
| Sulphonyl urea, n (%) | 216 (21.4) |
| Glinides, n (%) | 106 (10.5) |
| α-Glucosidase inhibitors, n (%) | 231 (22.9) |
| Thiazolidinediones, n (%) | 42 (4.2) |
| Biguanides, n (%) | 517 (51.2) |
| DPP-4 inhibitors, n (%) | 648 (64.2) |
| SGLT-2 inhibitors, n (%) | 162 (16.1) |
| GLP-1 receptor agonist, n (%) | 26 (2.6) |
Data are presented as means ± SD.
Abbreviations: BMI, body mass index.
3.1. Seasonal variation in HbA1c and BMI
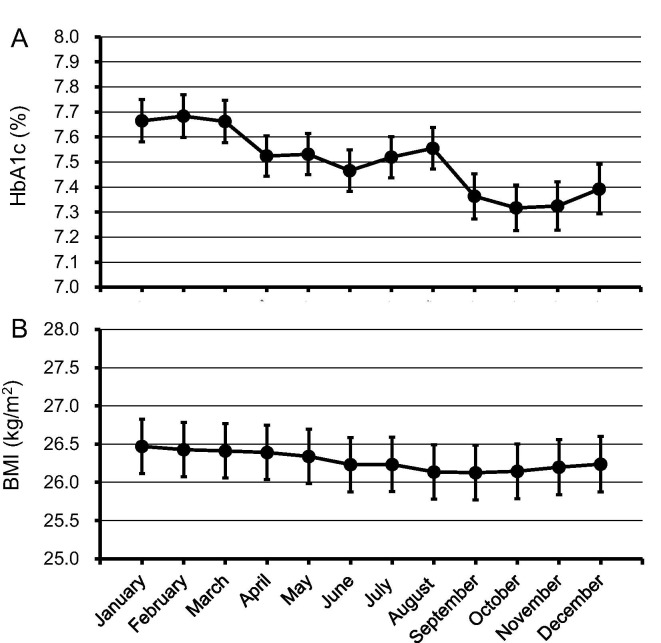
The linear mixed model adjusted for age, sex, BMI, insulin use, and year of measurement revealed that HbA1c values were the highest between January and March; subsequently, they fell gradually after April, reaching the lowest values in the period between September and November (Fig. 2 A). Using a similar model revealed that BMI values were highest in January and then gradually declined, reaching the lowest values in the period from August to October (Fig. 2B).
Fig. 2.
(A) Seasonal variation in HbA1c levels HbA1c values and their 95% confidence intervals for each month were calculated, using the mixed linear model adjusted for age, sex, body mass index, insulin use, and year of measurement. (B) Seasonal variation in body mass index Body mass index (BMI) values and their 95% confidence intervals for each month were calculated, using the mixed linear model adjusted for age, sex, HbA1c levels, insulin use, and year of measurement.
3.2. Impact of COVID-19 on HbA1c values and BMI
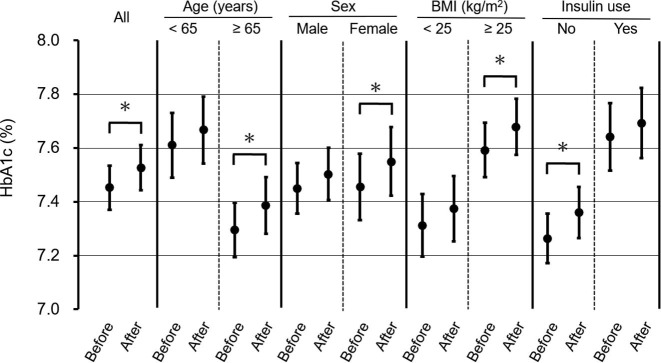
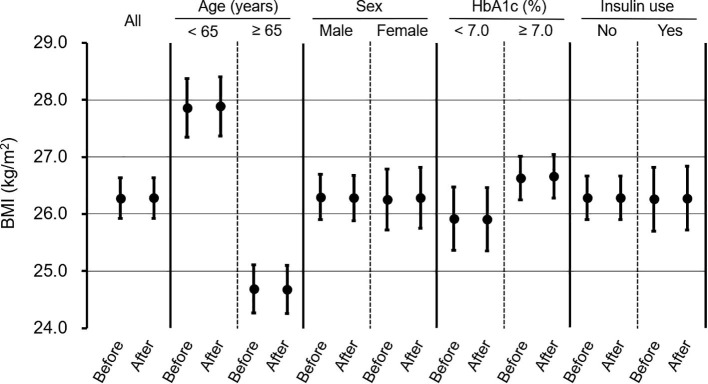
A model adjusted for sex, age, BMI, insulin use, and month and year of measurement revealed that HbA1c values significantly deteriorated after the state of emergency was declared in Japan (Fig. 3 ). Furthermore, HbA1c values significantly increased among women, patients aged ≥ 65 years and those with BMI of ≥ 25 kg/m2, as well as those that did not use insulin (Fig. 3). When we performed stratified analysis based on baseline HbA1c values (<7.0%, ≥ 7.0%), we observed that HbA1c values significantly increased after the state of emergency in patients with a baseline HbA1c level of < 7.0% (6.42% to 6.60%, p < 0.0001). In contrast, HbA1c values did not change in patients with a baseline HbA1c level of ≥ 7.0% (7.89% to 7.92%, p = 0.342). The group with baseline HbA1c < 7.0% included 24.9% insulin users, while the group with baseline HbA1c ≥ 7.0% included 34.4% insulin users. Meanwhile, BMI in patients with type 2 diabetes was the same before and after the state of emergency was declared (Fig. 4 ). Finally, change in BMI was similar in all subgroups (Fig. 4).
Fig. 3.
Analysis of HbA1c levels, stratified based on age, gender, body mass index, and insulin use HbA1c values and their 95% confidence intervals before and after the declaration of an emergency regarding COVID-19 pandemic were calculated, using the mixed linear model adjusted for age, sex, body mass index, insulin use, and month and year of measurement. *p-value < 0.05.
Fig. 4.
Analysis of body mass index, stratified based on age, gender, HbA1c levels, and insulin use Body mass index values and their 95% confidence intervals before and after the declaration of an emergency regarding COVID-19 pandemic were calculated, using the mixed linear model, adjusted for age, sex, HbA1c levels, insulin use, and month and year of measurement. *p-value < 0.05.
4. Discussion
In the present study, we investigated the impact of the COVID-19 pandemic on glycemic control among outpatients with type 2 diabetes. The present findings suggest that HbA1c values significantly worsened after the state of emergency was declared, while BMI remained stable in the study population.
Many countries worldwide have imposed lockdowns and other types of restrictions to contain the COVID-19 pandemic, including penalties for the violation of restrictions. In contrast, Japan did not impose a lockdown. Instead, the government declared the state of emergency due to the COVID-19 outbreak, calling on the nation to remain home and observe social distancing; the recommendations were neither enforceable nor subject to sanctions in cases of violations. Despite being relatively light, these measures dramatically changed the population’s behavior, affecting both physical and mental health [25], including diabetes self-management [26]. For example, Munekawa et al. reported that the COVID-19 pandemic changed the behavior of 183 patients with type 2 diabetes in Japan. Stress levels, overall dietary intake, snack consumption, and prepared food intake increased, while time dedicated to sleep and exercise decreased after the state of emergency was declared. These behavioral changes were associated with increases in HbA1c and BMI values [27]. In addition, Kishimoto et al. reported COVID-19-related behavioral changes among 168 patients with diabetes mellitus in Japan. Among them, 57 patients presented with HbA1c values deteriorating by > 0.2%, in contrast, 51 patients presented with HbA1c improved by > 0.2%. The former group was likely affected by increased snack and sweets consumption, changes to dietary and alcohol intake, and transition to teleworking and closure of sports facilities. Meanwhile, the latter group reported transitioning to a healthy diet and lowering their alcohol intake [28]. These findings suggest that behavioral management, including dietary habits, physical activity, medication and insulin adjustment, work routine, stress levels, and social relationships may affect glycemic control in patients with diabetes mellitus. However, the present study did not account for these behavioral parameters, which is a limitation of the present study.
In stratified analyses, we identified four subgroups particularly vulnerable to the worsening of glycemic control during the COVID-19 pandemic. First, HbA1c values significantly increased among women but not among men with diabetes mellitus. A recent study conducted in Japan reported that psychological distress associated with the COVID-19 pandemic was significantly larger among women than among men [29]. Women tend to be more affected by the social and economic consequences of a pandemic than do men [30]. In fact, a study of patients with diabetes mellitus in Denmark reported that women had more COVID-19-specific diabetes-related worries, suggesting getting infected may have greater consequences for women than it does for men with diabetes [31]. In the present study, women may have experienced worsened glycemic control due to high psychological stress associated with the pandemic.
Second, HbA1c values significantly increased in patients aged ≥ 65 years but not in younger patients. Recently, a survey of 1,600 community-dwelling older adults in Japan demonstrated decreased levels of physical activity and increased rates of sedentary behavior during the COVID-19 pandemic compared to before the pandemic [32]. Older adults are particularly vulnerable to COVID-19; the wish to avoid infection may have reduced their levels of activity. In addition, a survey of 5,000 adults in Japan demonstrated that the decrease in the frequency of outings and physical activity and the increase in the rate of sedentary behavior during the COVID-19 pandemic were greater among older than among younger adults [33]. Refraining from activity among older adults may have worsened glycemic control in the present patients aged ≥ 65 years.
Third, HbA1c values significantly increased in patients with BMI of ≥ 25 kg/m2 but not in those with BMI of < 25 kg/m2. A recent study from Spain has reported the effects of the COVID-19 pandemic on dietary habits of patients with type 2 diabetes, stratified by BMI [34]. Patients with BMI of 35–40 kg/m2 significantly increased sugary food consumption, in contrast to patients with BMI of 25–30 kg/m2 who did not show any changes in their sugary food consumption during the pandemic. Separate studies have indicated that people with obesity are unlikely to eat healthy or nutritionally balanced foods [35], [36]. Thus, in the present study, patients with BMI of ≥ 25 kg/m2 may have experienced the worsening of glycemic control due to changes in their dietary patterns.
Fourth, HbA1c values significantly increased in patients that were not receiving insulin treatment but not in those that were receiving such treatment. In Japan, SMBG or flash glucose monitoring (FGM) are currently reimbursed for patients with type 1 and 2 diabetes receiving daily insulin injections. These patients can monitor their glucose levels, using SMBG or FGM at home and adjust their insulin doses, dietary intake, and physical activity, as required. In contrast, SMBG or FGM are not reimbursed for patients with type 2 diabetes not treated with insulin. Thus, patients with type 2 diabetes receiving insulin treatment, most of whom use SMBG or FGM, may have been better placed to self-manage their disease than were their counterparts.
We further divided the subjects into two groups based on their baseline HbA1c level and observed the subsequent changes. The group with baseline HbA1c < 7.0% received less treatment with insulin than the group with baseline HbA1c ≥ 7.0%. The former group showed a significant increase in HbA1c after a state of emergency was introduced, while the latter group showed no change in HbA1c. As mentioned above, SMBG is reimbursed for insulin users in Japan. Thus, the group with baseline HbA1c ≥ 7.0% which includes many insulin users, could self-monitor blood glucose levels unlike the group with baseline HbA1c < 7.0%. Thus, the high HbA1c group may have been less susceptible to worsening blood glucose control during the COVID-19 pandemic. On the other hand, it is necessary to consider the “regression toward the mean”, which is observed when the baseline value is divided into two parts, and subsequent changes are observed.
Regular monitoring of HbA1c values is fundamental to the management of diabetes. Interruptions in HbA1c monitoring due to refraining from regular clinic visits and relying on telemedicine during the COVID-19 pandemic may have impaired diabetes management. A large national laboratory in the United States reported that the number of HbA1c measurements decreased by 66% between March and April 2020 compared to a similar period before the COVID-19 pandemic [37]. However, the number of patients who measured their HbA1c values at our clinic between March and April 2020 (1,164 patients) was the same as that observed between March and April 2019 (1,135 patients); meanwhile, the number of patients who requested telemedicine consultations was 30 (1.7%) in the present study sample. These findings suggest that the rate of measurements of HbA1c values at our clinic was not reduced during the pandemic, unlike in countries that imposed lockdowns [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23].
In the present study, BMI values remained unchanged after the state of emergency was introduced. Herein, we analyzed data collected until August 2020; therefore, the lack of change in BMI may be due to the short observation period. Previous studies on the impact of the COVID-19 pandemic on body weight in patients with diabetes mellitus were inconsistent, reporting an increase [7], no change [18], [21], [22], [23], and a decrease [19]. These previous studies also had short observation periods.
This study has some limitations. First, this was a single-center retrospective study; however, the sample size was much larger than those of the previous studies [6], [7], [9], [10], [11], [12], [13], [14], [15], [18], [19], [20], [22], [23] that examined the impact of the COVID-19 pandemic on glycemic control. Second, as this study was based at a university hospital, the sample may not be nationally representative. Third, only HbA1c values were used to assess glycemic control; thus, the presence of hypoglycemia or hyperglycemia detected by CGM was not evaluated. Fourth, several important clinical parameters affecting glycemic control were not assessed in this study (e.g., duration of diabetes and comorbidities). These confounding factors may have influenced the presented estimates.
In conclusion, we demonstrated that HbA1c values significantly worsened in patients with type 2 diabetes after the state of emergency due to COVID-19 was declared by the Japanese government. To our knowledge, this is the first study to report changes in HbA1c values during the COVID-19 pandemic in patients with type 2 diabetes in a country that did not impose a lockdown. Moreover, among patients with type 2 diabetes, women, patients aged ≥ 65 years, those with BMI of ≥ 25 kg/m2, and those that did not use insulin had difficulty maintaining glycemic control during the COVID-19 pandemic.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England). 2020;395:497–506. doi: 10.1016/s0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World-Health-Organization. Rolling updates on cornavirus disease (COVID-19) 2020 [31/03/2020]. . Avaiable from: http://wwwwhoint/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen. Last access 1/Dec/2020;10.1007/s13300-020-00829-7. https://doi.org/10.1007/s13300-020-00829-7.
- 3.Dyer O. Covid-19: Pandemic is having “severe” impact on non-communicable disease care, WHO survey finds. BMJ (Clinical research ed). 2020;369 doi: 10.1136/bmj.m2210. [DOI] [PubMed] [Google Scholar]
- 4.Jordan R.E., Adab P., Cheng K.K. Covid-19: risk factors for severe disease and death. BMJ (Clinical research ed). 2020;368 doi: 10.1136/bmj.m1198. [DOI] [PubMed] [Google Scholar]
- 5.Zhu L., She Z.G., Cheng X., Qin J.J., Zhang X.J., Cai J., et al. Association of Blood Glucose Control and Outcomes in Patients with COVID-19 and Pre-existing Type 2 Diabetes. Cell Metab. 2020;31(1068–77) doi: 10.1016/j.cmet.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernández E., Cortazar A., Bellido V. Impact of COVID-19 lockdown on glycemic control in patients with type 1 diabetes. Diabetes Res Clin Pract. 2020;166 doi: 10.1016/j.diabres.2020.108348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruissen MM, Regeer H, Landstra CP, Schroijen M, Jazet I, Nijhoff MF, et al. Increased stress, weight gain and less exercise in relation to glycemic control in people with type 1 and type 2 diabetes during the COVID-19 pandemic. BMJ open diabetes research & care. 2021;9. https://doi.org/10.1136/bmjdrc-2020-002035. [DOI] [PMC free article] [PubMed]
- 8.Potier L., Hansel B., Larger E., Gautier J.F., Carreira D., Assemien R., et al. Stay-at-Home Orders During the COVID-19 Pandemic, an Opportunity to Improve Glucose Control Through Behavioral Changes in Type 1 Diabetes. Diabetes Care. 2020 doi: 10.2337/dc20-2019. [DOI] [PubMed] [Google Scholar]
- 9.Aragona M., Rodia C., Bertolotto A., Campi F., Coppelli A., Giannarelli R., et al. Type 1 diabetes and COVID-19: The “lockdown effect”. Diabetes Res Clin Pract. 2020;170 doi: 10.1016/j.diabres.2020.108468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonora B.M., Boscari F., Avogaro A., Bruttomesso D., Fadini G.P. Glycaemic Control Among People with Type 1 Diabetes During Lockdown for the SARS-CoV-2 Outbreak in Italy. Diabetes therapy : research, treatment and education of diabetes and related disorders. 2020;11:1–11. doi: 10.1007/s13300-020-00829-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christoforidis A., Kavoura E., Nemtsa A., Pappa K., Dimitriadou M. Coronavirus lockdown effect on type 1 diabetes management οn children wearing insulin pump equipped with continuous glucose monitoring system. Diabetes Res Clin Pract. 2020;166 doi: 10.1016/j.diabres.2020.108307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mesa A, Viñals C, Pueyo Is, Roca D, Vidal M, Giménez M, et al. The impact of strict COVID-19 lockdown in Spain on glycemic profiles in patients with type 1 Diabetes prone to hypoglycemia using standalone continuous glucose monitoring. Diabetes research and clinical practice. 2020;167:108354. https://doi.org/10.1016/j.diabres.2020.108354. [DOI] [PMC free article] [PubMed]
- 13.Verma A., Rajput R., Verma S., Balania V.K.B., Jangra B. Impact of lockdown in COVID 19 on glycemic control in patients with type 1 Diabetes Mellitus. Diabetes & metabolic syndrome. 2020;14:1213–1216. doi: 10.1016/j.dsx.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khare J., Jindal S. Observational study on Effect of Lock Down due to COVID 19 on glycemic control in patients with Diabetes: Experience from Central India. Diabetes & metabolic syndrome. 2020;14:1571–1574. doi: 10.1016/j.dsx.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xue T, Li Q, Zhang Q, Lin W, Wen J, Li L, et al. Blood glucose levels in elderly subjects with type 2 diabetes during COVID-19 outbreak: a retrospective study in a single center. 2020;10.1101/2020.03.31.20048579 %J medRxiv:2020.03.31.20048579. https://doi.org/10.1101/2020.03.31.20048579 %J medRxiv.
- 16.Park S.D., Kim S.W., Moon J.S., Lee Y.Y., Cho N.H., Lee J.H., et al. on the Changes in Glycosylated Hemoglobin Level in People with Type 2 Diabetes Mellitus. Diabetes & metabolism journal. 2019;2020 doi: 10.4093/dmj.2020.0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khader M.A., Jabeen T., Namoju R. A cross sectional study reveals severe disruption in glycemic control in people with diabetes during and after lockdown in India. Diabetes & metabolic syndrome. 2020;14:1579–1584. doi: 10.1016/j.dsx.2020.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rastogi A., Hiteshi P., Bhansali A. Improved glycemic control amongst people with long-standing diabetes during COVID-19 lockdown: a prospective, observational, nested cohort study. International journal of diabetes in developing countries. 2020 doi: 10.1007/s13410-020-00880-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Psoma O., Papachristoforou E., Kountouri A., Balampanis K., Stergiou A., Lambadiari V., et al. Effect of COVID-19-associated lockdown on the metabolic control of patients with type 2 diabetes. J Diabetes Complications. 2020;34 doi: 10.1016/j.jdiacomp.2020.107756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biancalana E., Parolini F., Mengozzi A., Solini A. Short-term impact of COVID-19 lockdown on metabolic control of patients with well-controlled type 2 diabetes: a single-centre observational study. Acta Diabetol. 2020 doi: 10.1007/s00592-020-01637-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonora B.M., Morieri M.L., Avogaro A., Fadini G.P. The Toll of Lockdown Against COVID-19 on Diabetes Outpatient Care: Analysis From an Outbreak Area in Northeast Italy. Diabetes Care. 2020 doi: 10.2337/dc20-1872. [DOI] [PubMed] [Google Scholar]
- 22.Önmez A., Gamsızkan Z., Özdemir Ş., Kesikbaş E., Gökosmanoğlu F., Torun S., et al. The effect of COVID-19 lockdown on glycemic control in patients with type 2 diabetes mellitus in Turkey. Diabetes & metabolic syndrome. 2020;14:1963–1966. doi: 10.1016/j.dsx.2020.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghesquière L., Garabedian C., Drumez E., Lemaître M., Cazaubiel M., Bengler C., et al. Effects of COVID-19 pandemic lockdown on gestational diabetes mellitus: A retrospective study. Diabetes & metabolism. 2020 doi: 10.1016/j.diabet.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishii H., Suzuki H., Baba T., Nakamura K., Watanabe T. Seasonal variation of glycemic control in type 2 diabetic patients. Diabetes Care. 2001;24:1503. doi: 10.2337/diacare.24.8.1503. [DOI] [PubMed] [Google Scholar]
- 25.Hossain M.M., Sultana A., Purohit N. Mental health outcomes of quarantine and isolation for infection prevention: a systematic umbrella review of the global evidence. Epidemiology and health. 2020;42 doi: 10.4178/epih.e2020038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bornstein S.R., Rubino F., Khunti K., Mingrone G., Hopkins D., Birkenfeld A.L., et al. Practical recommendations for the management of diabetes in patients with COVID-19. The lancet Diabetes & endocrinology. 2020;8:546–550. doi: 10.1016/s2213-8587(20)30152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munekawa C., Hosomi Y., Hashimoto Y., Okamura T., Takahashi F., Kawano R., et al. pandemic on the lifestyle and glycemic control in patients with type 2 diabetes: a cross-section and retrospective cohort study. Endocr J. 2019;2020 doi: 10.1507/endocrj.EJ20-0426. [DOI] [PubMed] [Google Scholar]
- 28.Kishimoto M., Ishikawa T., Odawara M. Behavioral changes in patients with diabetes during the COVID-19 pandemic. Diabetology international. 2020 doi: 10.1007/s13340-020-00467-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sugaya N., Yamamoto T., Suzuki N., Uchiumi C. A real-time survey on the psychological impact of mild lockdown for COVID-19 in the Japanese population. Sci Data. 2020;7:372. doi: 10.1038/s41597-020-00714-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wenham C., Smith J., Morgan R. COVID-19: the gendered impacts of the outbreak. Lancet (London, England). 2020;395:846–848. doi: 10.1016/s0140-6736(20)30526-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joensen L.E., Madsen K.P., Holm L., Nielsen K.A., Rod M.H., Petersen A.A., et al. Diabetes and COVID-19: psychosocial consequences of the COVID-19 pandemic in people with diabetes in Denmark-what characterizes people with high levels of COVID-19-related worries? Diabetic medicine : a journal of the British Diabetic Association. 2020;37:1146–1154. doi: 10.1111/dme.14319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamada M., Kimura Y., Ishiyama D., Otobe Y., Suzuki M., Koyama S., et al. Effect of the COVID-19 Epidemic on Physical Activity in Community-Dwelling Older Adults in Japan: A Cross-Sectional Online Survey. The journal of nutrition, health & aging. 2020;24:948–950. doi: 10.1007/s12603-020-1424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamada K., Yamaguchi S., Sato K., Fuji T., Ohe T. The COVID-19 outbreak limits physical activities and increases sedentary behavior: A possible secondary public health crisis for the elderly. Journal of orthopaedic science : official journal of the Japanese Orthopaedic Association. 2020;25:1093–1094. doi: 10.1016/j.jos.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruiz-Roso M.B., Knott-Torcal C., Matilla-Escalante D.C., Garcimartín A., Sampedro-Nuñez M.A., Dávalos A., et al. COVID-19 Lockdown and Changes of the Dietary Pattern and Physical Activity Habits in a Cohort of Patients with Type 2 Diabetes Mellitus. Nutrients. 2020;12 doi: 10.3390/nu12082327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Drenowatz C., Shook R.P., Hand G.A., Hébert J.R., Blair S.N. The independent association between diet quality and body composition. Sci Rep. 2014;4:4928. doi: 10.1038/srep04928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sundararajan K., Campbell M.K., Choi Y.H., Sarma S. The relationship between diet quality and adult obesity: evidence from Canada. J Am Coll Nutr. 2014;33:1–17. doi: 10.1080/07315724.2013.848157. [DOI] [PubMed] [Google Scholar]
- 37.Fragala M.S., Kaufman H.W., Meigs J.B., Niles J.K., McPhaul M.J. Consequences of the COVID-19 Pandemic: Reduced Hemoglobin A1c Diabetes Monitoring. Population health management. 2020 doi: 10.1089/pop.2020.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]