Abstract
The unprecedented coronavirus disease 2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has made more than 125 million people infected and more than 2.7 million people dead globally. Airborne transmission has been recognized as one of the major transmission routes for SARS-CoV-2. This paper presents a systematic approach for evaluating the effectiveness of multi-scale IAQ control strategies in mitigating the infection risk in different scenarios. The IAQ control strategies across multiple scales from a whole building to rooms, and to cubical and personal microenvironments and breathing zone, are introduced, including elevated outdoor airflow rates, high-efficiency filters, advanced air distribution strategies, standalone air cleaning technologies, personal ventilation and face masks. The effectiveness of these strategies for reducing the risk of COVID-19 infection are evaluated for specific indoor spaces, including long-term care facility, school and college, meat plant, retail stores, hospital, office, correctional facility, hotel, restaurant, casino and transportation spaces like airplane, cruise ship, subway, bus and taxi, where airborne transmission are more likely to occur due to high occupancy densities. The baseline cases of these spaces are established according to the existing standards, guidelines or practices. Several integrated mitigation strategies are recommended and classified based on their relative cost and effort of implementation for each indoor space. They can be applied to help meet the current challenge of ongoing COVID-19, and provide better preparation for other possible epidemics and pandemics of airborne infectious diseases in the future.
Keywords: Airborne transmission, COVID-19, SARS-CoV-2, Wells-riley model, Mitigation strategy, IAQ
Graphical abstract
Nomenclature
- A
Room area (m2)
- ci
Conversion factor
- cv
Viral load in the sputum (RNA copies/mL)
- Cexhaust
Tracer gas concentration in the exhaust air
- Ci
Tracer gas concentration in the target location
- dp
Particle diameter (μm)
- fAirCleaner
Fraction of air cleaner operation time (%)
- frecirculated
Recirculated air fraction of the supply air for the HVAC system (%)
- fR
Fraction of time using a mask over the entire exposure period (%)
- fUV
Fraction of UVGI system operation time (%)
- H
Room height (m)
- I
Number of infectors
- kAirCleaner
Infectious particle removal rate by air cleaners (h−1)
- kdeposition
Infectious particle deposition rate (h−1)
- kinactivation
Pathogen natural inactivation rate in the air (h−1)
- kUV
Pathogen inactivation rate by UVGI systems (h−1)
- N
Total occupant number in the space
- NC
Number of new cases
- Nd
Particle number concentration (#/cm3)
- NS
Number of susceptible people
- p
Pulmonary ventilation rate (m3/h)
- P
Infection possibility (%)
- Pb
Overall infection probability for an occupant who visited multiple spaces (%)
- Pi
Infection probability in the i-th space (%)
- q
Infectious quantum generation rate per infector (h−1)
- R0
Basic reproduction numbers
- Ra
Required ventilation rate per room area (L/s·m2)
- RI
Fraction of infectious particle penetration through the infector's face mask (%)
- Rp
Required ventilation rate per person (L/s·p)
- RS
Fraction of infectious particle penetration through the susceptible individual's face mask (%)
- t
Exposure time (h)
- V
Room volume (m3)
- Vd
Volume of a single particle (cm3)
- εvent
Ventilation factor
- ηAirCleaner
Filter efficiency of air cleaner (%)
- ηfilter
Infectious particles filtration efficiency by the filters (%)
- ηI
Filtration efficiency of the infector's mask (%)
- ηS
Filtration efficiency of the susceptible individual's mask (%)
- λAirCleaner
Airflow rate of air cleaner (m3/h)
- λHVAC
Fresh air supply rate by the HVAC system (h−1)
- λoutdoor
Outdoor airflow rate of the HVAC system (h−1)
- λrecirculated
Recirculated airflow rate of the HVAC system (h−1)
- λsupply
Total supply airflow rate of the HVAC system (h−1)
- λvent
Equivalent ventilation rate (h−1)
- Λ
Equivalent fresh air change rate (h−1)
1. Introduction
The unprecedented coronavirus disease 2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has made more than 125 million people infected and more than 2.7 million people dead globally with more than half million deaths in U.S. alone [1]. Although a few biopharmaceutical companies have developed vaccines against COVID-19 [2,3], the worldwide distribution and use still require more time [4]. The COVID-19 pandemic is likely to last for a long period [5]. Recently, some new SARS-CoV-2 variants have been identified in some countries, bringing the world more unknown challenges to fight against the pandemic [[6], [7], [8], [9], [10]]. Minimizing the virus transmission is still essential in reducing the risk of COVID-19 infection.
There are typically three transmission routes of infectious respiratory viruses, including fomite route through contacts, droplet-borne route transmitted by medium or large droplets, and airborne route through aerosols that can remain suspended over a longer time [11]. The transmission of SARS-CoV-2 through fomite and droplet-borne routes was used to be considered as the main pathways, but more and more recent studies have revealed that the airborne transmission could be considered as the most relevant transmission route [[12], [13], [14], [15], [16], [17], [18], [19], [20], [21]], particularly in crowded and inadequately ventilated indoor spaces [[22], [23], [24]]. Fennelly [25] measured particle size distribution of infectious aerosols and observed that pathogens are more commonly found in small particles (<5 μm). Some studies have identified airborne transmission as a likely major pathway for asymptomatic transmission of SARS-CoV-2 [26,27] and the superspreading events [28]. Considering the substantial proportion of asymptomatic cases [[29], [30], [31]], airborne transmission is very important for analyzing the infection risk of COVID-19. Many public institutes, including WHO, U.S. CDC, PHAC and ASHRAE, have raised the concern on the airborne route of SARS-CoV-2 transmission [23,24,32,33].
People spend almost 90% of their time in indoor environments [[34], [35], [36], [37]]. Home-based outbreaks were found to be the dominant category (79.9%), followed by transportation-based outbreaks (34.0%) [38]. Nearly all superspreading events took place indoors [28]. Many indoor spaces have a high occupant density, but do not provide adequate fresh air [39], which increase the infection risk through airborne transmission. The transmission by airborne route was considered to greatly contribute to some reported outbreak events. For example, the SARS-CoV-2 spread among the members of the Skagit Valley Chorale during a weekly rehearsal eventually made 53 out of 61 members infected. Such a severe spread was highly suspected to be caused by the airborne transmission [14]. The outbreak event that happened in a Guangzhou restaurant was likely caused by the recirculated air, which carried infectious aerosols emitted by an index case [15,40]. A retrospective analysis for these two outbreak events also supported the airborne transmission of SARS-CoV-2 [41]. The outbreaks in a tour coach in Hunan province [16], a call center in Seoul [18] and a tour coach in Zhejiang province [17] also indicated the possibility of airborne transmission. It is increasingly clear and accepted that airborne transmission is an important contributor to the rapid and long-distance spreading of the SARS-CoV-2 [42].
Indoor air quality (IAQ) control strategies can be applied to reduce the infection risk of COVID-19 through airborne transmission [43,44]. Improving indoor ventilation systems, using air cleaning technologies and wearing masks can improve the IAQ and reduce the infection risk significantly. These strategies have been introduced and discussed in other published papers [[43], [44], [45]] and recommended by WHO [46], U.S. CDC [[47], [48], [49]] and ASHRAE [50].
A well-known mathematical model for estimating the infection risk through airborne transmission is the Wells-Riley model [51,52]. It assumes well-mixed air and a steady-state infectious particle concentration in a confined space. The estimation depends on the susceptible individual's inhalation exposure to the suspended pathogen generated by the infectors. The Wells-Riley model has been widely used to evaluate the airborne infection risk of respiratory diseases, such as influenza, tuberculosis, SARS-CoV-1, middle east respiratory syndrome (MERS), measles [[53], [54], [55], [56], [57]], and more recently, SARS-CoV-2. Dai and Zhao [45] used it to estimate the required ventilation rate in four scenarios ensuring a less than 1% infection probability. Harrichandra et al. [58] used it to estimate the airborne SARS-CoV-2 infection risk in nail salons. Multiple Wells-Riley model-based tools have been developed to help people evaluate the airborne transmission risk of COVID-19 [[59], [60], [61], [62], [63], [64]]. Considering the steady-state and well-mixed assumption for the indoor air, the Wells-Riley model has also been extended by some researchers to include unsteady exposure [65] and imperfect mixing [66,67]. In addition, the computational fluid dynamics (CFD) approach has also been conducted for studying the infectious particle dispersion in indoor environments and evaluating the infectious risk in according with the exposure dose [15]. But the CFD approach requires comprehensive information for the room and ventilation configurations, and the simulation process is usually time-consuming. Another widely used mathematical approach to model the transmission of COVID-19 is the Susceptible-Exposed-Infectious-Recovered (SEIR) epidemic disease model, which is usually used to estimate the epidemic disease transmission through all possible routes among a considerable number of populations (typically in a community scale) over a longer period (at least a few days) [68,69]. However, the SEIR model does not link the exposure directly to the risk of infection, and hence is not suitable for evaluating the effectiveness of IAQ strategies on airborne transmission.
This study aims to develop a systematic approach for evaluating the effectiveness of multi-scale IAQ control strategies in mitigating the infection risk in different building and transportation spaces. The IAQ control strategies across multiple scales will be introduced, including elevated outdoor air, high-efficiency filters, advanced air distribution strategies, standalone air cleaning technologies, personal ventilation, and face masks. The effectiveness of these strategies will be evaluated for specific indoor spaces, including long-term care facility, school and college, meat plant, retail stores, hospital, office, correctional facility, hotel, restaurant, casino and transportation spaces. The results can be applied to help handle the current challenge of ongoing COVID-19, as well as provide better preparation for possible epidemics or pandemics of airborne infectious diseases in the future.
2. Methodology
2.1. Risk estimation model
2.1.1. Wells-Riley model
The Wells-Riley model [70,71] is usually used to model the infection risk of airborne transmission in enclosed environments assuming a steady-state and well-mixed indoor environment. The infection possibility (P) is calculated as a function of the inhalation exposure dose [72], which depends on the number of pathogen carriers (i.e. infectors, I), the infectious quantum generation rate per infector (q), the fraction of infectious particle penetration through the face mask (R), pulmonary ventilation rate (p), exposure time (t) and the equivalent fresh air change rate in the room (Λ):
| (1) |
The fraction of infectious particle penetrated through the masks for susceptible (R S) and infected (R I) population depend on the mask filtration efficiency (η S or η I) and can be calculated by Eqs. (2), (3)), respectively. An additional fractional factor (f R) is multiplied by the original filtration efficiency of the mask to represent the fraction of time using a mask over the entire exposure period.
| (2) |
| (3) |
The equivalent air change rate (Λ) represents the equivalent supply flow rate of fresh air per unit volume of the room space. It depends on the equivalent ventilation air change rate (λ vent), pathogen inactivation rate by ultraviolet germicidal irradiation (UVGI) systems (k UV), infectious particle deposition rate (k deposition) and pathogen natural inactivation rate in the air (k inactivation):
| (4) |
The equivalent ventilation rate (λ vent) includes the fresh air supply rate by the heating, ventilation, and air conditioning (HVAC) system (λ HVAC) and standalone portable air cleaners (k AirCleaner). The original Wells-Riley model is based on the perfect-mixing assumption. However, indoor airflow patterns and mixing level are highly dependent on room configurations and air distributions. In order to evaluate the infection risk in imperfect-mixed scenarios, an additional ventilation factor (ε vent) is multiplied by the ventilation rate in the model, representing the dilution efficiency in a particular location compared to the perfect mixing ventilation. It can be estimated by comparing the tracer gas concentration in the target location (C i) and the concentration in the exhaust air (C exhaust) through Eq. (5), which is similar to the zone air distribution effectiveness in ASHRAE 62.1 [22]. It equals one for the perfect mixing condition. Spaces with more efficient air distribution (such as displacement ventilation) have a ventilation factor greater than one. A similar ventilation factor was also applied incorporating with the Wells-Riley model by Sun and Zhai [73]. The equivalent ventilation rate (λ vent) can be calculated by Eq. (6).
| (5) |
| (6) |
The fresh air supplied by the HVAC system (λ HVAC) includes the outdoor part and the recirculated part. The recirculated fresh air supply rate (Eq. (7)) depends on the recirculated airflow rate (λ recirculated) and the infectious particles filtration efficiency by the filters (η filter).
| (7) |
The total supply airflow rate of the ventilation system equals to the summary of outdoor airflow rate and the recirculated airflow rate, which can be calculated by
| (8) |
A portable air cleaner can supply additional fresh air. The infectious particle removal rate by air cleaners (k AirCleaner) can be estimated by its airflow rate (λ AirCleaner) and filter efficiency (η AirCleaner), or based on its clean air delivery rate (CADR) and room volume (V):
| (9) |
The pathogen removal rate by the UVGI system depends on the fraction of UVGI operation time (f UV) and the pathogen inactivation rate due to ultrafine (UV) irradiation (k UV). The infectious particle deposition rate (k deposition) relies on an approximate estimate of gravitational settling (Eq. (10)) [74], which depends on the particle diameter (d p) and room height (H). The possible impacts of environmental conditions on particle deposition [75] are not considered in this study.
| (10) |
2.1.2. Key parameters in the model
2.1.2.1. Infectious quantum generation rate per infector (q)
Quantum generation rate per infector (q) is a critical parameter in the Wells-Riley model. The magnitude of q depends on disease species, infector activities (e.g. breathing, coughing) and interventions (e.g. wearing masks), and may vary significantly case by case [70,76]. The value of q of a COVID-19 infector is currently not well established. It is believed to be close to the q of influenza and SARS-CoV-1 because their basic reproduction numbers (R 0) are close [45,[77], [78], [79], [80], [81], [82]]. Dai and Zhao [45] analyzed the statistical relationship between R 0 and q of other respiratory diseases, and estimated an approximate q between 14 and 48 h−1 for SARS-CoV-2 using the curve-fitting approach. Buonanno et al. [76] used a novel approach (Eq. (11)) for predicting the viral load emitted by a contagious subject based on the viral load in the sputum. It revealed that q could be lower than 1 h−1 in resting state and greater than 100 h−1 in light activity state.
| (11) |
The conversion factor c i is typically between 0.01 and 0.1 with a reported average value of 0.02 [76]. A typical q of 142 h−1 was estimated for a case who is speaking and doing light exercise [76]. Li et al. [15] estimated a q of 79.3 h−1 for an COVID-19 outbreak in a Guangzhou restaurant. Miller et al. [14] reported a q as high as 970 h−1 level for a super spreader during a chorale rehearsal. Buonanno et al. [41] proposed a new approach to evaluate the airborne transmission and performed a retrospective analysis for the Guangzhou restaurant case and the Skagit Valley Chorale case and revealed quantum generations of 61 h−1 and 341 h−1, respectively.
In this study, the infectious quantum generation rate is estimated based on the viral load model (Eq. (11)). Previous studies measured the viral load of COVID-19 patients and suggested that the viral load can typically reach 109 RNA copies/mL [76,[83], [84], [85], [86], [87], [88]], which is used in this study. A reported average value of 0.02 is applied as the c i in the model [76]. The highest droplet number concentration in Ref. [76] is adopted. The droplet volume calculation uses the geometric mean diameters for each particle size bin (i.e., 0.55 μm, 1.7 μm, and 5.5 μm for particles of 0.3–1 μm, 1–3 μm, and 3–10 μm, respectively [98]). Three different activity levels are considered: sedentary and light-intensity (breathing or whispering while seated or standing), moderate-intensity (speaking while seated or standing) and high-intensity (breathing or speaking while running or doing exercises). A Monte Carlo approach is applied to Eq. (11) to obtain the probability distribution of q for various age groups in various activities (Table 1 ), by adopting the probability density functions characteristic (normal distribution) of pulmonary rates in Table 3. The other parameters adopt constant values as presented above.
Table 1.
Estimated infectious quantum generation rate.
| Age group | Age [years] | Infectious quantum generation rate (Mean ± SD) [h−1] |
||
|---|---|---|---|---|
| Sedentary or light activities | Moderate-intensity activities | High-intensity activities | ||
| Children | <16 | 58 ± 31 | 251 ± 134 | 492 ± 270 |
| Adults | 16–61 | 58 ± 31 | 318 ± 177 | 610 ± 347 |
| Elders | >61 | 58 ± 31 | 305 ± 158 | 555 ± 307 |
Table 3.
Short-term pulmonary rates, by activity levels (adapted from Ref. [95]).
| Age group | Age [years] | Short-term pulmonary rates (Mean ± SD) [m3/h] |
||
|---|---|---|---|---|
| Sedentary or light activities | Moderate-intensity activities | High-intensity activities | ||
| Children | <16 | 0.3 ± 0.2 | 1.3 ± 0.85 | 2.5 ± 1.75 |
| Adults | 16–61 | 0.3 ± 0.2 | 1.6 ± 1.15 | 3.0 ± 2.3 |
| Elders | >61 | 0.3 ± 0.2 | 1.6 ± 1.0 | 2.8 ± 2.0 |
2.1.2.2. Size distribution of infectious particles
Considering the different aerodynamic features (e.g. deposition, filtration by filters) between the particles of different diameters, sufficient knowledge of droplet size is important for understanding virus transmission through the aerosol route. Researchers [57,89] have observed that most expelled droplets are in smaller size (typically below 2 μm). However, droplets in smaller diameters may contain less virus due to the smaller particle volume. When considering the droplet volume and droplet size distribution, larger droplets actually contain more pathogens [76,89].
Some studies have conducted measurements of virus size distribution in indoor environments, which accounts for a combination of all human respiratory activities that occur indoors. Stephens [57] reviewed such studies for influenza and estimated the size-resolved distribution of q using the data in literature [90]. It was observed that 15% of pathogens are in the 0.3–1 μm size range, 25% in the 1–3 μm size range and 60% in the 3–10 μm size range [57]. A report from CDPH [91] suggested the distribution of 20%, 30% and 50% for infectious particles in 0.3–1 μm, 1–3 μm and 3–10 μm, respectively. The published data [92,93] regarding the measured virus size distribution for SARS-CoV-2 in indoor environments are summarized in Table 2 (the original data were reclassified into bins of 0.3–1 μm, 1–3 μm and 3–10 μm in accordance with ASHRAE 52.2 [94]). Considering the virus size distribution in literature [57,[91], [92], [93]], the airborne infectious particle size distribution in this study is specified as probabilistic and assumed to follow the uniform distribution in each segment, i.e. 10-20% in 0.3–1 μm range, 20–30% in 1–3 μm, and 50–70% in 3–10 μm. It is generally consistent with the infectious particle size distribution defined in other modeling works [70,91]. The difference of size distribution between different age groups or activities is neglected [57].
Table 2.
| Particle aerodynamic diameter [μm] | Distribution of airborne SARS-CoV-2 across particle diameters [%] |
|||||
|---|---|---|---|---|---|---|
| Protective-apparel removal room A [92] | Protective-apparel removal room B [92] | Medical staff's office [92] | Patient room B [93] | Patient room C [93] | Average | |
| 0.3–1 | 96 | 58 | 24 | 0 | 0 | 36 |
| 1–3 | 2 | 5 | 18 | 41 | 50 | 23 |
| 3–10 | 2 | 37 | 59 | 59 | 50 | 41 |
2.1.2.3. Pulmonary ventilation rate (p)
The pulmonary ventilation rate in the Wells-Riley model is associated with the infectious aerosol dose inhaled by each susceptible person (Eq. (1)), and also related to the q of infectors (Eq. (11)). The short-term inhalation rates by activity level for people in different ages is shown in Table 3 . Three different activity levels are considered for each age group, including sedentary or light-intensity, moderate-intensity and high-intensity activities. The pulmonary rates are assumed to follow normal distributions.
2.1.2.4. Removal efficiency of filters for infectious particles (ηfilter)
The particle removal efficiency of filters used in the HVAC system is usually rated by minimum efficiency reporting values (MERVs). MERVs report a filter's ability to capture particles between 0.3 and 10 μm. The efficiency of MERV-rating filters for different particle size range is adapted from ASHRAE 52.2 [94]. The detailed information about the efficiency for each particle size is discussed in Supplemental Information. Considering the size distribution of infectious particles, the particle-size-weighted virus filtration efficiencies of different filters are presented in Table 4 .
Table 4.
Particle removal efficiency of different filters.
| MERV | Particle removal efficiency ηfilter [%] |
|||
|---|---|---|---|---|
| 0.3–1 μm | 1–3 μm | 3–10 μm | Particle-size-weighteda | |
| 1 | 0 | 0 | 10 | 5–7 |
| 2 | 0 | 0 | 10 | 5–7 |
| 3 | 0 | 0 | 10 | 5–7 |
| 4 | 0 | 0 | 10 | 5–7 |
| 5 | 3 | 17 | 20 | 16–18 |
| 6 | 3 | 17 | 35 | 23–28 |
| 7 | 9 | 17 | 50 | 32–39 |
| 8 | 9 | 20 | 70 | 43–54 |
| 9 | 9 | 35 | 85 | 55–67 |
| 10 | 9 | 50 | 85 | 59–70 |
| 11 | 20 | 65 | 85 | 66–74 |
| 12 | 35 | 80 | 90 | 76–82 |
| 13 | 50 | 90 | 90 | 82–86 |
| 14 | 75 | 90 | 90 | 87–88 |
| 15 | 85 | 90 | 90 | 89 |
| 16 | 95 | 95 | 95 | 95 |
| HEPAb | 99.9 | 99.9 | 99.9 | 99.9 |
Monte Carlo approach is implemented that adopts uniform probability distribution of particle sizes, i.e. 10-20% in 0.3–1 μm, 20–30% in 1–3 μm, and remaining 50–70% in 3–10 μm.
High-efficiency particulate air (HEPA) filters.
2.1.2.5. Removal efficiency of different masks on infectious particles (ηS and ηI)
Face masks provide air filtration at a personal level for wearers, which is a critical strategy for mitigating infection risk. Face masks can reduce the average emission rate by approximately 30%, 50% and 95% with cloth, surgical and N95 masks, respectively [96]. Konda et al. [97] measured the mask filtration efficiency for particles in different diameters (Table 5 ). The particle-size weighted removal efficiencies of different masks can be estimated based on the assumed infectious particle size distribution. The particle-size-weighted efficiency is around 32%, 44% and 95% for cloth, surgical and N95 masks, respectively.
Table 5.
Mask filtration efficiency for 0.3–1 μm, 1–3 μm, 3–10 μm and total particle-size-weighted average.
| Mask | Particle removal efficiency ηfilter [%] |
|||
|---|---|---|---|---|
| 0.3–1 μm | 1–3 μm | 3–10 μm | Particle-size-weightedc | |
| Cloth (cotton/silk, with gap)a | 27 | 33 | 34 | 32–33 |
| Surgical (with gap)a | 41 | 44 | 45 | 44 |
| N95b | 95 | 95 | 95 | 95 |
Average value of the data measured in Ref. [97].
Assuming 95% for all size ranges.
Monte Carlo approach is implemented that adopts uniform probability distribution of particle sizes, i.e. 10-20% in 0.3–1 μm, 20–30% in 1–3 μm, and remaining 50–70% in 3–10 μm.
2.1.2.6. Particle deposition
For the calculation of particle deposition, the same particle size bins as those used for HVAC filter MERV ratings are considered here to simplify the calculation, and values are calculated using the geometric mean diameters for each particle size bin (i.e., 0.55 μm, 1.7 μm, and 5.5 μm for particles of 0.3–1 μm, 1–3 μm, and 3–10 μm, respectively [98]) by Eq (10). Although it has been revealed that environmental conditions such as air temperature, humidity and airflow velocity may affect the travelling and deposition of exhaled aerosols, the conditions in most indoor environments are within a relatively narrower range. Therefore, typical indoor environments (23 °C, 50%RH and low airflow velocity) are assumed in this study. The possible impacts of environmental conditions on particle deposition are not considered.
2.1.2.7. Inactivation rate
van Doremalen et al. [99] observed an inactivation rate of 0.63 h−1 for SARS-CoV-2. Fears et al. [100] measured a nearly zero decay rate. Schuit et al. [101] revealed a mean decay rate of 0.48 h−1 without sunlight. Smither et al. [102] suggested a decay rate of 0.95 h−1 in aerosols at medium humidity condition and 0.24 h−1 at high humidity condition. Dabisch et al. [103] observed decay rates of 0.36 h−1 and 1.02 h−1 in the environment with room temperature and no sunlight. Therefore, the typical inactivation rate of SARS-CoV-2 aerosols at typical indoor temperature and humidity is generally between 0 and 1 h−1. Sunlight can possibly contribute greatly to the inactivation of SARS-CoV-2 [101,103], but is not considered in this study. A uniform distribution of inactivation rate between 0 and 1 h−1 is assumed in the model.
2.2. Definition of baseline cases
Significant percent of outbreaks have been reported in long-term care facility, manufacturing facility, correctional facility, school and college, healthcare facility and hospital, retail, restaurant and office facility, indicating these scenarios as hotspots for COVID-19 outbreaks [[104], [105], [106]]. The space layouts, occupant status and ventilation configurations of various spaces vary greatly. The researchers at the U.S. DOE and PNNL created prototypes of typical commercial buildings in accordance with ASHRAE 90.1 and IECC standards [[107], [108], [109]], which are used to define the baseline cases in this study. Design guidelines or real practices are adopted to define the baseline of other cases that were not defined by DOE and PNNL. The outbreaks tied to transportation spaces, such as airplanes, cruise ships and buses, have been widely reported [16,17,[110], [111], [112], [113], [114], [115], [116]], which will also be discussed.
The occupant number in the space is determined by the default occupant density in ASHRAE 62.1 [22] or the available data from literature or practices. Three different age groups are considered, including children, adults and elders. The occupant activities in different spaces are presented in ASHRAE 62.1 [22]. The occupant exposure duration is assigned based on the most typical practices in real scenarios. The required outdoor ventilation rate can be calculated based on the data in ASHRAE 62.1 [22], which depends on the space area and occupant number (Eq. (12)). For other cases, the data from literature or typical practices are used to define the baseline ventilation rate. The baseline definitions for all studied spaces are listed in Table 6 , while more detailed information can be found in Supplemental Information.
| (12) |
Table 6.
Configurations of baseline cases.
| Scenarioa | Space type | Space layout |
Occupant status |
Ventilation configuration |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Area [m2] | Height [m] | Density [#/100m2] | Number [person] | Duration [h] | Activity levelc [−] | Rp [L/s·p] | Ra [L/s·m2] | Ventilation rated [L/s] | |||
| Long-term care facility | Bedroom (double resident) | 36.8 | 3.0 | / | 2 | 11 | Elder Sed. | 2.5 | 0.3 | 16.0 | |
| Dining room | 70.0 | 3.0 | / | 20 | 2 | Elder Mod. | 3.8 | 0.9 | 139.0 | ||
| Living room | 50.0 | 3.0 | 10 | 5 | 2 | Elder Sed. | 2.5 | 0.3 | 27.5 | ||
| Physical therapy roomb | 23.2 | 3.0 | 20 | 5 | 2 | Elder Mod. | 5 | 0.3 | 32.0 | ||
| Educational | K-12 | Classroom | 99.0 | 4.0 | 35 | 35 | 4e | Child Sed.e | 5 | 0.6 | 234.4 |
| Library | 840.1 | 4.0 | 10 | 84 | 1 | Child Sed. | 2.5 | 0.6 | 714.1 | ||
| Cafeteria/dining room | 624.0 | 4.0 | 100 | 624 | 1 | Child Mod. | 3.8 | 0.9 | 2932.8 | ||
| Gym | 1976.2 | 8.0 | 7 | 138 | 1 | Child High | 10 | 0.9 | 3158.6 | ||
| College | Classroom (small) | 51.5 | 3.0 | / | 25 | 2 | Adult Sed. | 5 | 0.6 | 155.9 | |
| Classroom (large) | 150.0 | 4.0 | / | 96 | 2 | Adult Sed. | 5 | 0.6 | 570.0 | ||
| Library (public study area) | 338.6 | 6.0 | / | 96 | 2 | Adult Sed. | 2.5 | 0.6 | 443.2 | ||
| Auditorium | 1134.0 | 14.6 | / | 1500 | 2 | Adult Sed. | 3.8 | 0.3 | 6040.2 | ||
| Computer lab | 84.3 | 4.0 | / | 38 | 2 | Adult Sed. | 5 | 0.6 | 240.6 | ||
| Dining hall | 573.5 | 4.0 | 100 | 574 | 1 | Adult Mod. | 3.8 | 0.9 | 2697.4 | ||
| Study lounge | 84.3 | 4.0 | / | 21 | 2 | Adult Sed. | 2.5 | 0.6 | 103.1 | ||
| Gym (fitness area) | 256.0 | 8.0 | / | 60 | 2 | Adult High | 10 | 0.9 | 830.4 | ||
| Resident hall (bedroom) | 21.5 | 3.0 | / | 2 | 8 | Adult Sed. | 2.5 | 0.3 | 11.5 | ||
| Greek house (social gathering) | 50.0 | 3.0 | / | 20 | 4 | Adult Mod. | 2.5 | 0.3 | 65.0 | ||
| Manufacturing facility | Meat plant | Processing room (dense) | 434.0 | 4.0 | / | 108 | 8 | Adult Mod. | 5.0 | 0.9 | 930.6 |
| Processing room (sparse) | 434.0 | 4.0 | / | 27 | 8 | Adult Mod. | 5.0 | 0.9 | 525.6 | ||
| Retail | Standalone | Core shopping space | 1600.4 | 6.0 | 15 | 240 | 1 | Adult Mod. | 3.8 | 0.6 | 1872.2 |
| Strip mall | Store (large) | 348.4 | 5.2 | 8 | 28 | 1 | Adult Mod. | 3.8 | 0.3 | 210.9 | |
| Store (small) | 174.2 | 5.2 | 8 | 14 | 1 | Adult Mod. | 3.8 | 0.3 | 105.5 | ||
| Healthcare facility | Hospital | Operating room | 55.7 | 4.3 | / | 3 | 4 | Adult Sed. | / | / | 198.2 |
| Patient room (patient + doctor) | 20.9 | 4.3 | / | 2 | 1 | Adult Sed. | / | / | 49.6 | ||
| Physical therapy room | 487.6 | 4.3 | / | 26 | 2 | Adult Mod. | / | / | 186.0 | ||
| Dining room | 696.5 | 4.3 | / | 75 | 1 | Adult Mod. | / | / | 902.4 | ||
| Lobby | 1474.3 | 4.3 | / | 21 | 1 | Adult Mod. | / | / | 499.3 | ||
| Office | Medium | Open plan office | 191.9 | 2.7 | 5 | 10 | 8 | Adult Sed. | 2.5 | 0.3 | 82.6 |
| Enclosed office | 42.3 | 2.7 | 5 | 2 | 8 | Adult Sed. | 2.5 | 0.3 | 17.7 | ||
| Conference room | 43.2 | 2.7 | 50 | 22 | 2 | Adult Sed. | 2.5 | 0.3 | 68.0 | ||
| Lounge | 89.6 | 2.7 | 50 | 45 | 1 | Adult Sed. | 2.5 | 0.6 | 166.3 | ||
| Correctional facility | Prison | Housing (double resident cell) | 10.0 | 3.0 | / | 2 | 8 | Adult Sed. | 2.5 | 0.6 | 11.0 |
| Housing (dormitory) | 160.0 | 3.0 | 25 | 40 | 8 | Adult Sed. | 2.5 | 0.6 | 196.0 | ||
| Dayroom | 160.0 | 6.0 | 30 | 48 | 12 | Adult Sed. | 2.5 | 0.3 | 168.0 | ||
| Lodging | Hotel | Guest room/bedroom | 39.0 | 3.0 | / | 2 | 8 | Adult Sed. | 2.5 | 0.3 | 16.7 |
| Banquet/dining room | 331.7 | 3.0 | 70 | 232 | 2 | Adult Mod. | 3.8 | 0.9 | 1180.1 | ||
| Lobby | 1308.2 | 4.0 | 30 | 392 | 1 | Adult Mod. | 3.8 | 0.3 | 1882.1 | ||
| Other public facilities | Restaurant | Dining room (ordinary) | 371.7 | 3.0 | 70 | 260 | 1 | Adult Mod. | 3.8 | 0.9 | 1322.5 |
| Dining room (fast-food) | 116.1 | 3.0 | 70 | 81 | 0.5 | Adult Mod. | 3.8 | 0.9 | 412.3 | ||
| Religious | Worship hall | 204.0 | 4.0 | / | 200 | 2 | Adult Sed. | 2.5 | 0.3 | 561.2 | |
| Casino | Poker room | 253.1 | 4.0 | 120 | 304 | 4 | Adult Mod. | 3.8 | 0.9 | 1383.0 | |
| Transportation spaces | Airplane | Cabin | 101.8 | 2.2 | / | 160 | 4 | Adult Sed. | 3.5 | / | 560 |
| Cruise ship | Guest room (double resident) | 17.0 | 3.0 | / | 2 | 8 | Adult Sed. | 2.5 | 0.3 | 10.1 | |
| Casino | 635.5 | 3.0 | 120 | 763 | 4 | Adult Mod. | 3.8 | 0.9 | 3471.4 | ||
| Cafeteria/Bistro | 80.0 | 3.0 | 100 | 80 | 2 | Adult Mod. | 3.8 | 0.9 | 376.0 | ||
| Subway | Cabin | 40.7 | 2.5 | / | 176 | 0.5 | Adult Sed. | / | / | 480.5 | |
| Bus | Transit bus | 30 | 2.5 | / | 60 | 0.5 | Adult Sed. | / | / | 210 | |
| Tour coach | 30 | 2.5 | / | 50 | 2 | Adult Sed. | / | / | 175 | ||
| School/shuttle bus | 15.4 | 2.2 | / | 16 | 0.5 | Child Sed. | / | / | 56 | ||
| Taxi | Cabin | 3 | 1.3 | / | 4 | 0.5 | Adult Sed. | / | / | 41.2 | |
References for baseline definition of various scenarios: Long-term care facility [117,118]; K-12 school [108]; College [[119], [120], [121], [122]]; Meat plant [123]; Retail facility [108]; Hospital [108]; Office [124,125]; Correctional facility from typical practices; Hotel [108]; Restaurant [108]; Religious facility [126]; Casino [127]; Airplane [128,129]; Cruise ship [130,131]; Subway [132]; Transit bus [133]; Tour coach [133]; School bus [133,134]; Taxi from typical practices.
Physical therapy rooms existed in buildings where residents require medical cares, such as nursing homes.
Sed.: sedentary; Mod.: moderate-intensity activities; High: high-intensity activities.
Outdoor air ventilation rate (calculated by Eq. (12)).
When a teacher is infector, the exposure duration is 1 h and the activity level for the infector is adult moderate-intensity level.
2.3. Multi-scale IAQ strategies for mitigating airborne transmission
The U.S. CDC [[47], [48], [49]] and WHO [46] have provided guidance for infection risk mitigation strategies in different spaces. Some of the strategies mainly focus on mitigating the airborne transmission, including improving ventilation rate, upgrading filters, using air cleaners or upper-room UVGI systems and wearing masks. ASHRAE [50] introduced some air cleaning technologies to mitigate the disease transmission through aerosols. Zhang [43] and Morawska et al. [44] introduced similar control strategies for mitigating infection risks in spaces like office and classroom.
The current IAQ control strategies can be roughly divided into three categories, i.e. source control, ventilation and air cleaning [43]. For the control of SARS-CoV-2 generation sources, it can be achieved through isolating infectors or preventing the virus emission from them. Therefore, limiting the occupant number or applying intermittent occupancy [135], and removing the expelled aerosols locally by wearing masks or applying local air exhaust [[136], [137], [138]], are the strategies that possibly could control the virus source [43,139]. For the room ventilation, it aims to supplying sufficient clean air and delivering it to occupants. The ventilation can be improved by increasing the airflow or outdoor air fraction of the ventilation system, optimizing the room air distribution to avoid cross-infection [43]. The air cleaning strategies involve applying air filtration or purification in the ventilation duct, locally in the room, and for the breathing zone. For the ventilation system, high-efficiency filters can be installed in the duct. Standalone air cleaners and upper-room UVGI systems can provide additional clean air. Properly wearing masks is another air cleaning approach for the susceptible individuals since the infectious particles can be filtered by masks before being inhaled [139].
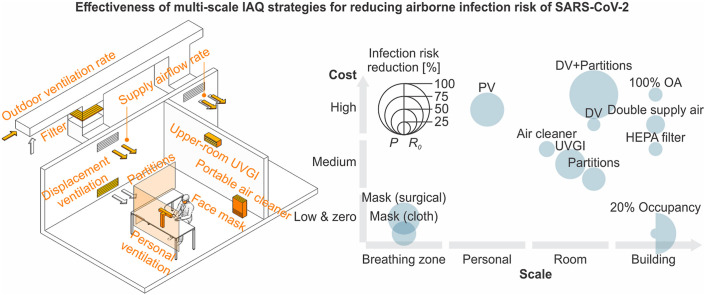
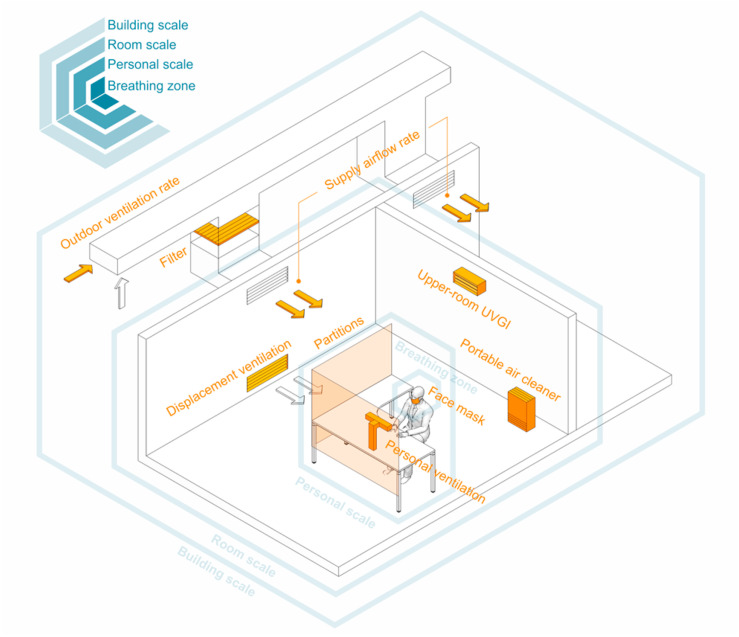
These IAQ control strategies can be implemented in different scales, from a whole building, to a room or space, to personal microenvironments and the breathing zone, which could result in different performance. Generally, the strategies implemented in building scale can mitigate infection for a considerable number of occupants but are not able to control the airborne transmission locally in a room or the breathing zone. Besides, these control strategies may cause more penalties on building energy consumption. The strategies in room, personal and breathing zone scales are more likely to mitigate the infection effectively since they are closely associated with the quantity of inhaled infectious particles. The possible IAQ control strategies in different scales are presented in Table 7 and illustrated in Fig. 1 .
Table 7.
Possible IAQ control strategies in different scales.
| Strategies | Scales |
|||
|---|---|---|---|---|
| Building | Room | Personal | Breathing zone | |
| Source control |
|
|
|
|
| Ventilation |
|
|
|
|
| Air cleaning |
|
|
|
|
Fig. 1.
Possible control strategies in different scales.
The performance of each IAQ control strategy is analyzed in this study based on the Wells-Riley model. For a baseline case (see Table 8 ), the mixing ventilation with reference ventilation rate defined in Table 6 is applied. Persily and Gorfain [140] suggested that the average outdoor air fraction for building ventilation system to be around 25%, which is used as the baseline outdoor air fraction. It is consistent with the configurations in other studies [57]. A MERV 8 filter is used for the recirculated air of the ventilation system for the baseline case, in accordance with ASHRAE 62.1 [22], except for the hospital operating room and the airplane cabin where the ventilation system typically use HEPA filters [141]. Standalone air cleaners and upper-room devices are not used in the baseline case. People in the baseline scenario do not wear any mask.
Table 8.
Configurations of baseline and proposed cases.
| Strategies | Baseline | Proposed | |
|---|---|---|---|
| Ventilation system | Ventilation rate (outdoor air) |
|
|
| Total supply airflow rate |
|
|
|
| Air distributiona |
|
|
|
| Filter |
|
|
|
| Standalone devices | Portable air cleaners |
|
|
| Upper-room UVGI system |
|
|
|
| PPE | Mask |
|
|
Mixing ventilation: εvent = 1; Displacement ventilation: εvent = 1.2 to 2; Semi-open space with partitions installed: εvent = 2 to 3; Displacement ventilation with partitions installed: εvent = 14 to 100; Personal ventilation: εvent = 1.4 to 10; all assuming uniform distribution.
HEPA filter is used in the baseline cases of hospital operating room and airplane cabin. All other spaces use MERV 8 filter as the baseline setup.
Equivalent ACH = 12 h−1 for mixing ventilation and equivalent ACH = 9.6 h−1 for displacement ventilation.
The proposed cases are simulated by applying different control strategies to the baseline cases. Elevated ventilation rates are analyzed, including 50%, 75% and 100% outdoor air based on the baseline total supply airflow rate. Increased total supply airflow rate are also tested, including 50% and 100% more supply flow rate within the limitation of system capacity. Advanced air distribution methods are tested. The ventilation factor (ε vent) of displacement ventilation may vary greatly, depending on exact air distribution patterns. It generally has the potential to reduce the pollutant concentration in the occupied zone by a factor of 1.2–2, which is close to the zone air distribution effectiveness for displacement ventilation in ASHRAE 62.1 [22]. In this study, the ventilation factor (ε vent) of displacement ventilation is assumed to be as a uniform distribution between 1.2 and 2. Installing partitions in the room to form semi-open spaces can provide a ventilation factor between 2 and 3 [142]. A more effective approach is to integrate the displacement ventilation and partitions, with a uniform distributed ε vent between 14 and 100 [143]. Personal ventilation has the potential to improve the air quality by a factor of 1.4–10 [144], which is close to the air distribution effectiveness in ASHRAE 62.1 [22]. However, personal ventilation and combined displacement ventilation and partitions strategy likely require professional design before being used. Higher-efficiency filters are also considered, including MERV 13 and HEPA filters as recommended by ASHRAE [50,145].
Portable air cleaners are widely used nowadays. Zhao et al. [146] reviewed the most popular air cleaners and suggested a median CADR level of 361 m3/h. Liu et al. [147] observed similar results. The use of air cleaners usually depends on the room scale or occupant number. Liu et al. [147] reviewed the CADR and typical applying area of air cleaners. The CADR per square meter is roughly between 6 and 16 m3/h. The Association of Home Appliance Manufacturers (AHAM) recommended a minimum CADR of 12 m3/h per square meter when selecting an air cleaner for home use [148], which is in accordance with the U.S. EPA's guide [149]. Therefore, the CADR of the air cleaners in this study is determined by the room area with a reference of 12 m3/h per square meter.
Upper-room UVGI systems are considered a supplement to other control strategies. The effectiveness of UVGI system on respiratory diseases has been studied [[150], [151], [152], [153]]. Appropriate use of upper-room UVGI system can inactivate airborne virus significantly. A well-designed upper-room UVGI system can typically provide equivalent 12 to 16 air changes per hour to the room [150,151]. In this study, the efficacy of an upper-room UVGI system is assumed to be 12 h−1. The performance of virus inactivation by the upper-room UVGI system also depends on the room air distribution. Displacement ventilation may reduce the efficiency as the residence time of the virus in the irradiated zone decreases. The UVGI system is assumed to provide 9.6 air changes per hour (80%) when integrated with a displacement ventilation system [154].
Face masks, including cloth, surgical and N95 masks, can filter droplets significantly and protect the susceptible individuals [155]. People in the U.S. are more likely to wear cloth masks than surgical and N95 masks [156]. But surgical and N95 masks can provide better protection. Germany recently requires all individuals in the country to wear medical-grade face masks [157]. In this study, cloth masks will be considered as the most typical personal protective equipment (PPE), but surgical and N95 masks will be discussed as well. However, it may not be possible for people to wear masks in some scenarios, e.g. dining, sleeping or performing high-intensity activities [158].
2.4. Model setting and simulations
A stochastic Monte Carlo approach is applied to consider for the possible variation of the input data and increase the representativeness of the estimation since the unknown parameters in the model can vary greatly. The simulation trials for each case are 100,000. The probability distribution of each unknown parameter has been introduced in above sections. The Monte Carlo approach is performed for estimating the probability distributions of quantum generation rates and infection probabilities. This study focuses on airborne transmission due to asymptomatic infectors. The estimated proportion of active asymptomatic patients (around 1% based on current data [[159], [160], [161], [162]]) is used to assign the number of index patients in the target space with a minimum of one infector. The number of new infection cases can be estimated based on the infection probability and the susceptible occupant number. The basic reproduction number (R 0) of COVID-19 due to airborne transmission [163] can be simply represented as
| (13) |
R0 indicates the disease spreading in population. When R 0 < 1, the disease dies out; when R 0 > 1, an epidemic occurs in the population [68]. In order to control the COVID-19 spreading through airborne transmission, both infection probability and R 0 should be minimized in the target space.
The infection probabilities are estimated space by space. The infection probability of an occupant in the building depends on the risks of the spaces he/she visited, which indicates the importance of the occupant's behavior and scheduling. Assuming that the infection risks in different spaces are independent from each other, the overall infection probability for an occupant in the building (P b) can be simply estimated based on the infection probabilities in all spaces he/she visited (P i) by
| (14) |
3. Results and discussion
3.1. Baseline infection risk in different spaces
The estimated infection probability and R 0 of SARS-CoV-2 for the baseline spaces are shown in Table 9 . It is observed that the standard deviations (SD) of infection probability and R 0 are relatively high, indicating a wide variation for both parameters. The infection probabilities over 10% and the R 0 over 5 are marked in the table, indicating the spaces with high infection risks. Generally, under the baseline conditions (without any control strategy), almost all the studied scenarios are facing very high infection risks.
Table 9.
Infection probability and basic reproduction number (R0) for baseline cases.
| Scenario | Space type | Infection probability [%] |
R0 [-] |
|||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Long-term care facility | Bedroom (double) | 50.0 | 29.9 | 0.5 | 0.3 | |
| Dining room | 48.2 | 28.7 | 9.2 | 5.5 | ||
| Living room | 10.6 | 9.4 | 0.4 | 0.4 | ||
| Physical therapy room | 78.3 | 29.0 | 3.1 | 1.2 | ||
| Educational | K-12 | Classroom (between students) | 3.8 | 3.6 | 1.3 | 1.2 |
| Classroom (teacher is the infector) | 13.2 | 12.0 | 4.5 | 4.1 | ||
| Library | 0.3 | 0.2 | 0.2 | 0.2 | ||
| Cafeteria/dining room | 10.1 | 8.9 | 8.9 | 7.9 | ||
| Gym | 8.3 | 7.7 | 5.6 | 5.2 | ||
| College | Classroom (small) | 3.1 | 2.9 | 0.7 | 0.7 | |
| Classroom (large) | 0.9 | 0.8 | 0.8 | 0.8 | ||
| Library (public study area) | 0.9 | 0.8 | 0.8 | 0.8 | ||
| Auditorium | 1.1 | 1.1 | 1.1 | 1.1 | ||
| Computer lab | 2.0 | 1.9 | 0.7 | 0.7 | ||
| Dining hall | 14.6 | 12.8 | 13.8 | 12.1 | ||
| Study lounge | 3.8 | 3.6 | 0.8 | 0.7 | ||
| Gym (fitness area) | 38.0 | 27.0 | 22.4 | 15.9 | ||
| Resident hall (bedroom) | 52.5 | 30.4 | 0.5 | 0.3 | ||
| Greek house (social gathering) | 77.5 | 30.2 | 14.7 | 5.7 | ||
| Manufacturing facility | Meat plant | Processing room (dense) | 53.7 | 31.2 | 28.5 | 16.5 |
| Processing room (sparse) | 47.8 | 29.9 | 12.4 | 7.8 | ||
| Retail | Standalone | Core shopping space | 8.4 | 7.8 | 6.6 | 6.2 |
| Strip mall | Store (large) | 17.8 | 15.4 | 4.8 | 4.1 | |
| Store (small) | 30.1 | 23.0 | 3.9 | 3.0 | ||
| Healthcare facility | Hospital | Operating rooma | 1.0 | 0.9 | 0.0 | 0.0 |
| Patient room (patient + doctor) | 4.5 | 4.2 | 0.0 | 0.0 | ||
| Physical therapy room | 29.0 | 22.4 | 7.2 | 5.6 | ||
| Dining room | 6.4 | 6.1 | 4.8 | 4.5 | ||
| Lobby | 6.7 | 6.5 | 1.3 | 1.3 | ||
| Office | Medium | Open plan office | 12.6 | 11.0 | 1.1 | 1.0 |
| Enclosed office | 39.8 | 26.7 | 0.4 | 0.3 | ||
| Conference room | 6.2 | 5.7 | 1.3 | 1.2 | ||
| Lounge | 1.4 | 1.3 | 0.6 | 0.6 | ||
| Correctional facility | Prison | Housing (double resident cell) | 59.5 | 31.4 | 0.6 | 0.3 |
| Housing (dormitory) | 7.9 | 7.2 | 3.1 | 2.8 | ||
| Dayroom | 11.6 | 10.2 | 5.4 | 4.8 | ||
| Lodging | Hotel | Guest room/bedroom | 41.0 | 27.2 | 0.4 | 0.3 |
| Banquet/dining room | 27.8 | 21.5 | 21.2 | 16.4 | ||
| Lobby | 12.0 | 10.8 | 11.6 | 10.5 | ||
| Other public facilities | Restaurant | Dining room (ordinary) | 14.7 | 12.8 | 12.6 | 11.0 |
| Dining room (fast-food) | 8.4 | 7.8 | 6.7 | 6.2 | ||
| Religious | Worship hall | 1.7 | 1.6 | 1.7 | 1.6 | |
| Casino | Poker room | 47.0 | 29.6 | 35.2 | 22.2 | |
| Transportation spaces | Airplane | Cabin | 2.3 | 2.2 | 1.8 | 1.7 |
| Cruise ship | Guest room (double resident) | 56.7 | 31.1 | 0.6 | 0.3 | |
| Casino | 41.7 | 27.9 | 39.4 | 26.4 | ||
| Cafeteria/Bistro | 20.3 | 16.3 | 16.0 | 12.9 | ||
| Subway | Cabin | 0.6 | 0.5 | 0.5 | 0.5 | |
| Bus | Transit bus | 0.6 | 0.6 | 0.4 | 0.4 | |
| Tour coach | 2.9 | 2.7 | 1.4 | 1.3 | ||
| School/shuttle bus | 2.2 | 2.1 | 0.3 | 0.3 | ||
| Taxi | Cabin | 3.2 | 3.0 | 0.1 | 0.1 | |
Surgical masks are used.
Spaces in long-term care facilities, colleges, meat plants, hotels, restaurants, casinos and cruise ships are facing considerably higher infection probabilities (over 30%) and have a higher potential to result in a serious outbreak or even superspreading event (R 0 > 10). It is generally consistent with the reported cases [[104], [105], [106]]. For each scenario, the risks in different spaces can vary greatly. The spaces in the long-term care facility generally have high infection probabilities, particularly physical therapy room, dining room and bedroom. Considering the dense occupancy and high infection probability in the dining room, the disease is more likely to spread out in the dining room (R 0 = 9.2). The occupant living in the same bedroom with the index patient, is exposed to a considerable infection probability (50%). But the disease is unlikely to spread out across bedrooms (R 0 < 1), unless more people get infected during the gathering in other public spaces like dining room or therapy room.
The infection probabilities in K-12 school spaces are not as high as the probabilities in the long-term care facility. The dining space, gym and classroom have higher infection probabilities. However, considering the occupant number in K-12 schools, the disease has more potential to spread out among the students, particularly in the dining space. The infection risk in the library is low, probably due to the low occupancy and relatively better ventilation. For the virus spreading in classrooms, it can be observed that a teacher (13.2%) is much more likely to spread the disease to the class members, than a student patient (3.8%) because of the teacher's higher q. The prediction is in agreement with the results from the other studies [164,165].
The infection risks in the college spaces vary significantly. Studying spaces, such as classrooms, library and computer labs, are generally less hazardous than the non-studying spaces. It is highly consistent with the reported outbreaks in colleges [[166], [167], [168], [169], [170], [171]]. Gym, dining hall and Greek house in a social gathering are exposed to very high infection risks and can result in superspreading events (high R 0). Considering that people are less likely to wear masks in these spaces, reopening is not recommended for these spaces unless high-efficiency infection mitigation strategies are applied. Based on Eq. (14), for a student who only visited studying spaces, the overall infection probability is around 10%, which is much lower than the probability for a student who also visited dining hall, gym, and Greek house (89%). The infection probability in bedrooms of the resident hall is extremely high when living with an infector (52.5%). However, the disease is unlikely to spread across the bedrooms in the resident hall, unless infection happened in other public spaces.
The employees in the processing room of meat plant meet great challenges of COVID-19 infection. Superspreading event is likely to happen in the processing room with dense employees (R 0 over 28). It is consistent with the frequently reported superspreading events in meat plants [123,[172], [173], [174], [175], [176], [177], [178], [179], [180]]. The retails also have high infection risks. The infection probability in smaller store is much higher than the probability in larger store and mall. But the mall and large store usually have more customers, thus have more potential to spread out the disease to more people. Physical therapy room, dining room and lobby are the spaces with higher risks in the hospital. The infection probabilities in patient room are relatively low, because of the high ventilation rate per occupant. The infection probability in the operating room is very low (1%) due to the use of surgical masks. The office spaces generally have high infection probabilities, particularly the enclosed office (39.8%). But the disease is more likely to spread out to more people in other public spaces with more occupants, such as open plan offices and conference rooms (higher R 0).
The susceptible individual who lives together with an infector in the same double-residence cell in the prison is highly possible to get infected (nearly 60% infection probability). Then the COVID-19 can spread to more people during the gathering activities in the dayroom (R 0 = 5.4). Another type of housing unit, i.e. dormitory, are more likely to spread the disease than the housing cell, likely due to its high occupancy. Considering that the actual living environment in the prison may be even worse, e.g. more crowded dormitory or cell, longer gathering time and inadequate ventilation, the infection risks in real prison scenarios could be much higher. It can partially explain the frequent outbreaks in the prison [[181], [182], [183], [184], [185], [186]], as well as the severe superspreading phenomenon [28].
For a hotel, the susceptible individual faces extremely high infection probability in the bedroom where an infector occupied, but the disease is more likely to spread to other people during the contacts in a public space (e.g. dining room or cafeteria). Banquet room has the highest potential to spread out the disease, due to the high-intensity activities and dense occupancy. Restaurants have high infection risks and can spread out the disease significantly, as encountered in the reported actual cases [187]. The infection probability in the worship hall of a religious building is not as high as the probability in other public spaces. But the disease can still spread out among the people in the worship hall (R 0 = 1.7). The infection risk in the casino is almost the highest among all the scenarios. Considering the high-intensity activities and crowded occupancy, the casino has a very high potential for superspreading outbreak (R 0 = 35.2).
For the transportation spaces, cruise ship basically has the highest infection risk since it typically contains casinos and dining spaces, where the disease can spread out readily. The infection probability in airplanes is similar to the probability in tour coaches and school buses, around 2–3%. Due to the considerable passengers presented in the airplane and tour coach, the disease can spread out in these two scenarios (R 0 = 1.8 for airplane and R 0 = 1.4 for tour coach). The infection risks during shorter transits are typically lower than the risks during longer transits.
3.2. Effectiveness of multi-scale control strategies
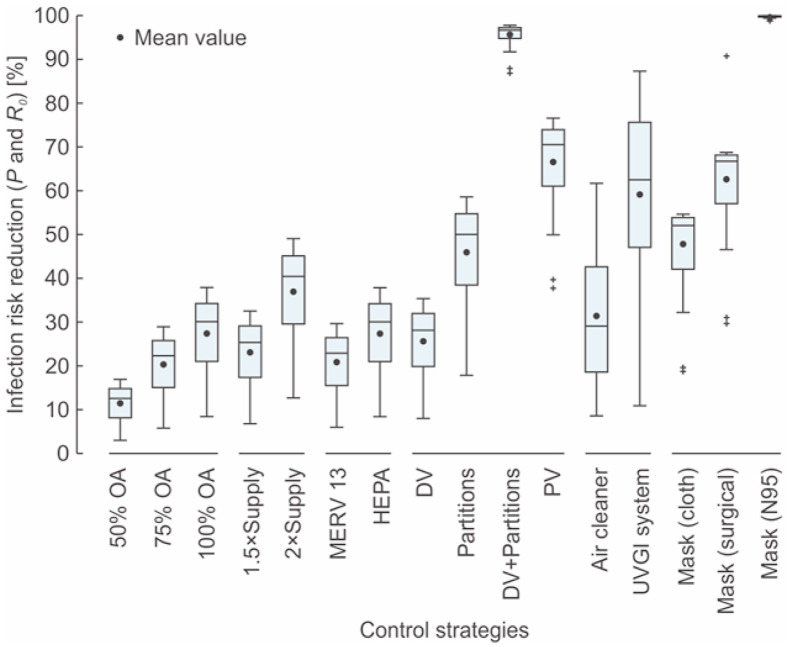
The infection risks, including infection probability and R 0, in different spaces using various control strategies are calculated. The reductions of the infection probability and R 0 for the same case should be same since the susceptible number is the same. The infection risk and R 0 reduction of each individual control strategy are determined relative to the baseline case by the model simulation (Fig. 2 ). The ventilation system with more outdoor air can reduce more infection risk. An average risk reduction of 27% can be achieved when using 100% outdoor air (OA). Increasing the total supply airflow rate can reduce considerable infection risk as well. Doubling the total supply airflow rate can reduce around 37% risk in average. A higher-efficiency filter in the ventilation system can supply more cleaned air. A HEPA filter can reduce equivalent infection risk to the strategy applying 100% outdoor air. Room air distributions can greatly impact the infection risk. Displacement ventilation (DV) can reduce average 26% infection risk, while installing partitions can reduce around 46% risk. Personal ventilation (PV) can reduce more infection risk, average 67%. Integrating displacement ventilation and partitions can maximize the ventilation factor with an average 96% of infection risk reduction. The impacts of the standalone air cleaning technologies vary greatly in various spaces, from below 10% risk reduction to over 85%. The average risk reduction for air cleaners is around 31%, and the reduction for the upper-room UVGI system is around 59%. Wearing cloth masks can generally reduce considerable infection risk (average 48%), while surgical and N95 masks can reduce more risks, i.e. average 63% and 99%, respectively.
Fig. 2.
Risk reduction distribution of the mean infection probabilities and R0 in different spaces.
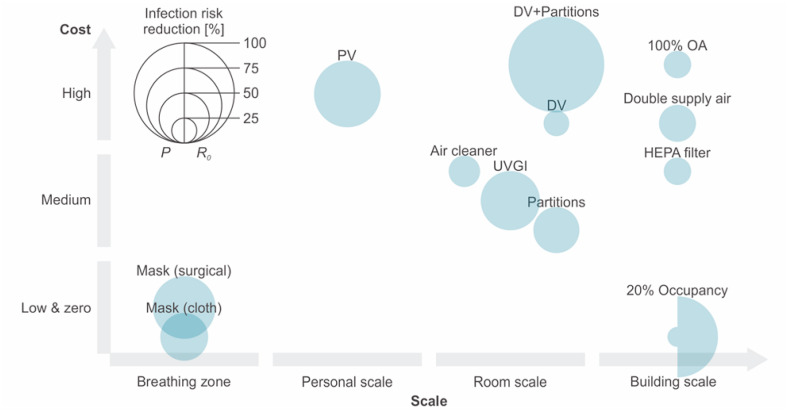
In addition to the infection risk reduction potential, the functional scale and cost should also be considered. Some control strategies, such as high-efficiency filters for the ventilation system, may not be able to provide as high as infection risk reduction as strategies like applying personal ventilation or wearing masks. But it can improve the IAQ for the whole building, indicating that it is functional for a larger scale of susceptible individuals. Besides, some control strategies may have a higher cost, e.g. personal ventilation or displacement ventilation, which makes these strategies difficult to implement in many buildings. There have been many investigations regarding the effectiveness of possible control strategies for mitigating the infection risk of COVID-19. However, most of these studies conducted qualitative analyses or did not consider the costs and functional scales of the strategies [[43], [44], [45]]. The mean infection risk reduction potentials and approximate costs of control strategies in different scales are shown in Fig. 3 . Air cleaners, UVGI systems, partition installation and HEPA filters are considered as medium-cost strategies because they usually require the purchase of a few devices or materials. Personal and displacement ventilations are considered as high-cost strategies because they require an upgrade for the entire ventilation system or some parts of it. The ventilation systems using 100% outdoor air or doubling total also considered high-cost strategies, considering the additional energy consumption for heating or cooling the elevated outdoor air.
Fig. 3.
Infection risk reduction potentials and costs of control strategies in different scales.
It can be observed from Fig. 3 that integrating the displacement ventilation and partitions can reduce more infection risk in the room scale but can be costly as well, while using displacement ventilation alone has a much lower reduction in infection risk. Personal ventilation can considerably reduce the infection risk at the personal scale but has a high cost. Personal ventilation can only be used in scenarios where occupants are more stationary. Using air cleaners has a medium cost and a moderate potential for infection risk reduction. Higher CADR for air cleaners is encouraged. Installing partitions and using the UVGI systems can have higher risk reduction potentials. Wearing mask has a moderate infection risk reduction potential and a low cost but can only work for the breathing zone. The effectiveness of the occupancy restriction strategy is illustrated in Fig. 3 as well. The 20% occupancy restriction does not change the infection probability greatly (average 20%) since at least one infector is assumed to exist in the space. But the R 0 can be reduced significantly (average 81%) as less susceptible people will be infected. Therefore, occupancy restriction is a strategy that can significantly contribute to the control of disease spreading in the population. Another potentially effective and low-cost room-scale strategy on source control is the intermittent occupancy strategy, which implements intermittent breaks in room occupancy [135]. It requires all occupants to leave the room periodically and the room occupancy time should be reduced as much as possible. It was reported that asking students to leave the room during the 15min break after a 35min class can reduce 35% inhaled pathogen compared to when the students stayed in the room during the break [135]. However, this study adopts the steady-state model, which does not consider the variation of indoor infectious particle concentration over time. The intermittent occupancy strategy is therefore not discussed in this study.
Although some control strategies in building and room scales may have higher costs and relatively smaller risk reduction potential, it does not mean that the infection risk mitigation should primarily rely on the strategies in smaller scales. Control strategies in building and room scales can reduce the “background” infection risk in the confined space, while strategies in personal scale and breathing zone can provide additional local protection to occupants. Considering that personal ventilation and mask wearing are unlikely to be implemented in some scenarios, enhanced ventilation is therefore essential. Besides, the high risk reduction potential of personal ventilation and mask wearing depends on the proper use of them. For example, improper use of face masks or any violation of mask wearing guidelines can put the susceptible occupants at risk. Therefore, when designing risk mitigating strategies, the risk reduction, cost, and scale of the control strategy should be considered comprehensively, rather than just focusing on one aspect.
3.3. Integrated effects of multiple control strategies
A single control strategy usually cannot provide adequate protection for occupants. It is necessary to integrate multiple strategies. Engineering control strategies at the building scale, i.e. elevated outdoor air, increased total supply air and higher-efficiency filters, should be applied as the primary mitigation strategy because they protect a large number of occupants and can be more reliably applied. Room-scale control strategies, including air distribution strategies and standalone air cleaning devices, should be applied to supplement the building level strategies. Personal ventilation system can be adopted to further reduce the risk of infection at the personal scale. Administrative strategies, such as restricting occupancy, must be implemented when the engineering controls cannot provide a safe environment. Face masks are essential because they reduce virus emission and protect individuals at the personal level.
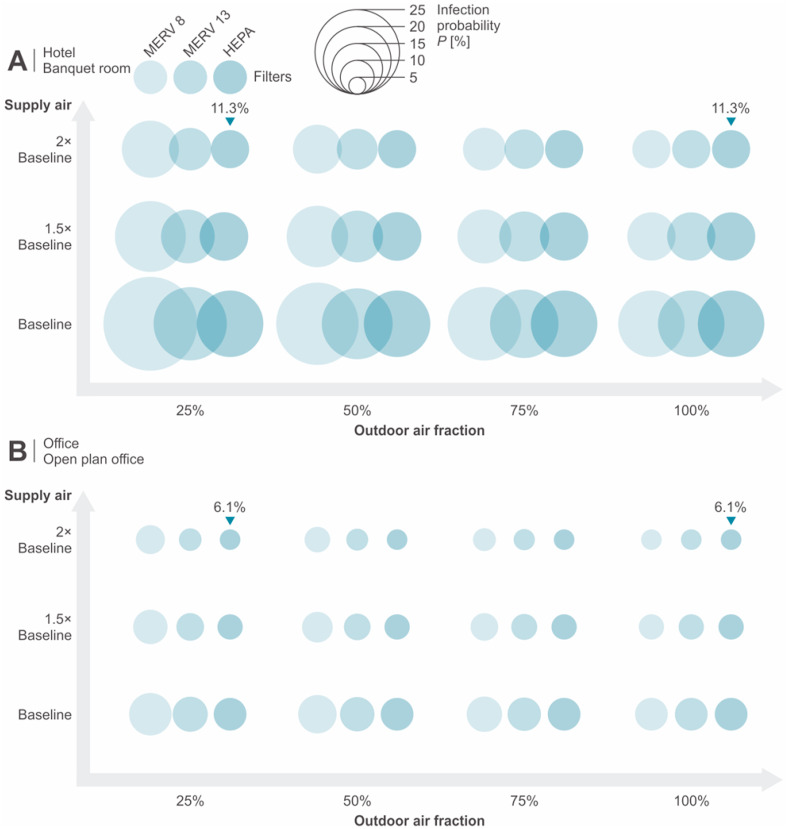
For the control strategies at the building scale, applying HEPA filters is equivalent to the effectiveness of adopting 100% outdoor air. When HEPA filters are adopted in the ventilation system, elevating the outdoor air does not improve the air quality significantly. Doubling the total supply air within the system capacity can further increase the clean air supply. Fig. 4 demonstrates the mean infection probabilities for three enhanced ventilation strategies in hotel banquet room and open plan office. The case applying double supply airflow rate, 25% outdoor air and HEPA filter has the same level of infection risk as the case using 100% outdoor air. Considering the substantial costs due to energy consumption penalty, the strategy of applying 100% outdoor air is not favored, unless HEPA filters cannot be used in the system. Therefore, the favored strategy for the ventilation system is the integration of double supply air and HEPA filter.
Fig. 4.
Infection probability of integrating multiple ventilation system strategies in (A) hotel banquet room and (B) open plan office.
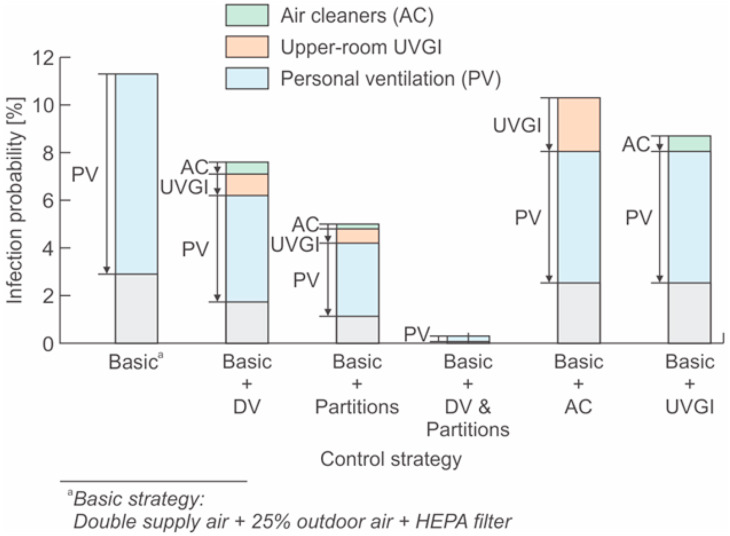
The integrated effectiveness of room-scale air distribution strategies and standalone air cleaning technologies in the hotel banquet room is illustrated in Fig. 5 as an example. Partitions are favored than the displacement ventilation system due to the higher risk reduction potential, especially considering the higher cost of a displacement system. Combining displacement ventilation and partitions strategy can significantly reduce the infection probability. But professional design has to be done to better organize the air distribution for maximizing its effectiveness. Installing partitions is likely a more practical strategy. Portable air cleaners (AC) and upper-room UVGI systems can provide additional clean air but may not be so effective when the indoor infection probability is already at a lower level. However, for those spaces that cannot install partitions or apply displacement ventilations, air cleaners and UVGI system can mitigate more risks. When room-scale strategies still cannot maintain a safe indoor environment, personal ventilation system should be considered whenever possible. Further, administrative restriction on indoor occupancy needs to be conducted to reduce the disease spreading among people for those cases with high R 0. Face masks are considered as the final protection where hazards are not well controlled.
Fig. 5.
Infection probabilities of integrating different air distribution strategies and standalone air cleaning technologies in the hotel banquet room.
The proposed approach has also been applied to the studied building and transportation spaces to find the best practice for risk mitigation. The acceptable level of the COVID-19 infection probability is not clear. In this study, the mean infection probability should be reduced to a level below 0.1%, which is also a risk reference accepted by Buonanno et al. [41]. The mean value of R 0 should be lower than one. The possible practices for mitigating the infection risks to the target level for each space are shown in Table 10 . The technology marked by a black dot is a technology that is applied in the target space. Otherwise, it means the technology is not implemented. The slash sign in the table indicates the technologies which are not practical to be applied in the target space. It is observed from the results that most spaces require double supply air, HEPA filter, displacement ventilation, partitions, air cleaners and UVGI systems. Dining spaces and open plan offices may need personal ventilation to provide additional clean air. Some other spaces, such as meat plant processing room and casino spaces, must request the occupants to wear masks. The housing spaces (e.g. bedroom, resident hall and resident cell) in various scenarios generally have a high infection probability since some strategies cannot be applied in bedrooms. The social gathering of college students in Greek houses has the highest risk even though all possible control strategies are applied. Therefore, social gathering in Greek houses should not be performed at the current stage. For most spaces, the infection risk can be reduced to be less than 0.1% using the integrated engineering control strategies according to the modeling. Wearing face masks can further reduce the risk, especially in public spaces where close contact with an infector can result in a higher risk.
Table 10.
Possible practice for mitigating the infection risk to the target level.
| Scenario | Space type | Control strategya |
Infection riskf |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Double supply | 100% OAb | HEPA filter | DVc | Part.d | AC | UVGI | PV | 20% Occup.e | Mask | P [%] | R0 [-] | |||
| Long-term care facility | Bedroom (double) | ● | ● | ● | / | ● | ● | / | / | / | 10.3 | 0.1 | ||
| Dining room | ● | ● | ● | ● | ● | ● | ● | / | 0.17 | <0.1 | ||||
| Living room | ● | ● | ● | ● | ● | ● | ● | / | <0.1 | <0.1 | ||||
| Physical therapy room | ● | ● | ● | ● | ● | ● | / | / | ● | 1.4 | <0.1 | |||
| Educational | K-12 | Classroom (between students) | ● | ● | ● | ● | ● | ● | <0.1 | <0.1 | ||||
| Classroom (teacher is the infector) | ● | ● | ● | ● | ● | ● | ● | <0.1 | <0.1 | |||||
| Library | ● | ● | ● | <0.1 | <0.1 | |||||||||
| Cafeteria/dining room | ● | ● | ● | ● | ● | ● | <0.1 | <0.1 | ||||||
| Gym | ● | ● | ● | ● | ● | ● | / | / | <0.1 | <0.1 | ||||
| College | Classroom (small) | ● | ● | ● | ● | ● | ● | <0.1 | <0.1 | |||||
| Classroom (large) | ● | ● | ● | ● | ● | <0.1 | <0.1 | |||||||
| Library (public study area) | ● | ● | ● | ● | ● | <0.1 | <0.1 | |||||||
| Auditorium | ● | ● | ● | ● | ● | ● | <0.1 | <0.1 | ||||||
| Computer lab | ● | ● | ● | ● | ● | ● | <0.1 | <0.1 | ||||||
| Dining hall | ● | ● | ● | ● | ● | ● | ● | / | <0.1 | <0.1 | ||||
| Study lounge | ● | ● | ● | ● | ● | ● | <0.1 | <0.1 | ||||||
| Gym (fitness area) | ● | ● | ● | ● | ● | ● | / | / | 0.5 | 0.3 | ||||
| Resident hall (bedroom) | ● | ● | ● | / | ● | ● | / | / | / | 11.8 | 0.1 | |||
| Greek house (social gathering) | ● | ● | ● | / | ● | ● | / | / | 36.3 | 6.9 | ||||
| Manufacturing facility | Meat plant | Processing room (dense) | ● | ● | ● | ● | ● | ● | ● | ● | 0.1 | <0.1 | ||
| Processing room (sparse) | ● | ● | ● | ● | ● | ● | ● | ● | <0.1 | <0.1 | ||||
| Retail | Standalone | Core shopping space | ● | ● | ● | / | ● | ● | / | ● | 0.5 | 0.4 | ||
| Strip mall | Store (large) | ● | ● | ● | / | ● | ● | / | ● | 1.0 | 0.3 | |||
| Store (small) | ● | ● | ● | / | ● | ● | / | ● | 2.0 | 0.3 | ||||
| Healthcare facility | Hospital | Operating room | ● | ● | ● | / | ● | ● | / | / | ●g | 0.3 | <0.1 | |
| Patient room (patient + doctor) | ● | ● | ● | ● | ● | ● | / | <0.1 | <0.1 | |||||
| Physical therapy room | ● | ● | ● | ● | ● | ● | / | ● | 0.2 | <0.1 | ||||
| Dining room | ● | ● | ● | ● | ● | ● | / | <0.1 | <0.1 | |||||
| Lobby | ● | ● | ● | / | ● | ● | / | ● | 0.3 | <0.1 | ||||
| Office | Medium | Open plan office | ● | ● | ● | ● | ● | ● | ● | <0.1 | <0.1 | |||
| Enclosed office | ● | ● | ● | ● | ● | ● | ● | / | ● | <0.1 | <0.1 | |||
| Conference room | ● | ● | ● | ● | ● | ● | <0.1 | <0.1 | ||||||
| Lounge | ● | ● | ● | ● | ● | ● | <0.1 | <0.1 | ||||||
| Correctional facility | Prison | Housing (double resident cell) | ● | ● | ● | / | ● | ● | / | / | / | 17.3 | 0.2 | |
| Housing (dormitory) | ● | ● | ● | / | ● | ● | / | / | 1.2 | 0.5 | ||||
| Dayroom | ● | ● | ● | ● | ● | ● | ● | <0.1 | <0.1 | |||||
| Lodging | Hotel | Guest room/bedroom | ● | ● | ● | / | ● | ● | / | / | / | 7.3 | <0.1 | |
| Banquet/dining room | ● | ● | ● | ● | ● | ● | ● | / | <0.1 | <0.1 | ||||
| Lobby | ● | ● | ● | / | ● | ● | / | ● | 0.9 | 0.8 | ||||
| Other public facilities | Restaurant | Dining room (ordinary) | ● | ● | ● | ● | ● | ● | ● | / | <0.1 | <0.1 | ||
| Dining room (fast-food) | ● | ● | ● | ● | ● | ● | / | <0.1 | <0.1 | |||||
| Religious | Worship hall | ● | ● | ● | ● | ● | ● | <0.1 | <0.1 | |||||
| Casino | Poker room | ● | ● | ● | ● | ● | ● | ● | ● | <0.1 | <0.1 | |||
| Transportation spaces | Airplane | Cabin | ● | ● | ● | ● | / | / | <0.1 | <0.1 | ||||
| Cruise ship | Guest room (double resident) | ● | ● | ● | / | ● | ● | / | / | / | 14.0 | 0.1 | ||
| Casino | ● | ● | ● | ● | ● | ● | ● | ● | <0.1 | <0.1 | ||||
| Cafeteria/Bistro | ● | ● | ● | ● | ● | ● | ● | <0.1 | <0.1 | |||||
| Subway | Cabin | ● | ● | / | / | / | / | / | ● | <0.1 | <0.1 | |||
| Bus | Transit bus | ● | ● | / | / | / | / | / | ● | <0.1 | <0.1 | |||
| Tour coach | ● | ● | ● | ● | / | / | <0.1 | <0.1 | ||||||
| School/shuttle bus | ● | ● | ● | ● | / | / | <0.1 | <0.1 | ||||||
| Taxi | Cabin | ● | ● | / | ● | / | / | ● | / | <0.1 | <0.1 | |||
Black dot (●) means the technology is used; Slash sign (/) indicates the technologies which are not practical to be applied in the target space.
100% outdoor air (OA) strategy can be an alternative for HEPA filter, whenever HEPA filter cannot be used in the system.
Displacement ventilation (DV) is less favored compared to the use of partitions, air cleaners or UVGI systems, since it requires professional design for maximizing its effectiveness.
Part.: using partitions.
20% Occup.: restricting the room occupancy to 20% of its capacity for cases with high R0.
Infection probability P should be lower than 0.1% and R0 should be lower than 1. Those cases that cannot achieve the target risk level are marked.
Surgical masks are used.
4. Summary and conclusions
A modified SARS-CoV-2 airborne transmission model has been applied to systematically evaluate multi-scale IAQ control strategies in mitigating the indoor infection risk in buildings and vehicles. Probability functions of essential model parameters were determined based on a comprehensive review of available literature to provide inputs for the stochastic simulation by the Mont Carlo method. Control strategies at building, room, personal and breathing-zone scales, including elevated outdoor air, high-efficiency filters, advanced room air distribution, standalone room air cleaning, personal ventilation and face masks, were analyzed. It was observed that under the established baseline conditions, the spaces in long-term care facilities, colleges, meat plants, hotels, restaurants, casinos and cruise ships would face considerable infection probabilities (over 30%) and have a higher potential to spread out among people (R 0 > 10).
The effectiveness of the control strategies has been analyzed for each space type in terms of risk reduction relative to the baseline conditions. More outdoor ventilation air can reduce more infection risk as expected. An average of 27% reduction of infection risk can be achieved with 100% outdoor air (OA) for the ventilation system. A HEPA filter for recirculated air can have the equivalent reduction. Doubling total supply airflow rate can reduce the infection risk by approximately 37% in average. Room air distributions can significantly impact the infection risk. Displacement ventilation can reduce the infection risk by 26%, while installing partitions can reduce more risk, around 46%. An average of 96% infection risk reduction can be achieved by integrating displacement ventilation and partitions. Personal ventilation can reduce the infection risk by 67%. The average risk reduction by air cleaners is around 31%, and the average reduction for the upper-room UVGI system is 59%. Wearing cloth masks can generally reduce considerable infection risk (average 48%), while surgical and N95 masks can reduce even more infection risk (63% and 99%, respectively).
When designing the risk mitigation strategies, the effectiveness, cost, and scale of the control strategy should be considered comprehensively. Enhanced ventilation at the building scale generally has higher costs due to increase in energy consumption for air heating or cooling but can mitigate risk for a large number of occupants and can be more reliably applied. Standalone room air cleaning is effective with moderate cost. Combining advanced air distribution (such as displacement and personal ventilation) with semi-open partition has high risk reduction potential, but with relatively high costs. Control strategies at building and room scales can reduce the “background” infection risk indoors, while strategies at personal scale and breathing zone (face masks) can provide additional needed local and personal protections.
To mitigate the infection risk to the target level (P < 0.1%, R 0 < 1), most spaces require doubling supply air, HEPA filter, displacement ventilation, partitions, air cleaners and UVGI systems. Dining spaces and open plan offices may need personal ventilation to provide additional clean air. Some other spaces, such as meat plant processing room and casino spaces, need to require occupants to wear masks. The housing spaces generally have a high infection probability since many strategies cannot be applied in bedrooms. The social gathering of college students in Greek houses has the highest risk. Face masks are always recommended to further reduce the risk, especially due to possible close contact with an infector.
The well-mixing assumption of the model does not consider the detailed local airflow pattern in the room, which can impact the local infection risk for each individual in the room. Further studies considering the detailed indoor air distribution are needed for better understanding the local infection risk within a space. The definitions of the baseline cases in this study represent the most typical configurations. But the actual risk in a specific space highly depends on its specific configurations, considering that the building spaces/rooms may vary greatly case by case. The present work only provides a reference evaluation for each scenario. Besides, a more quantitative analysis of the cost and energy consumption of different control strategies is also needed in further studies.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research was jointly sponsored by Carrier Corporation and Syracuse University.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.buildenv.2021.107926.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.WHO, WHO Coronavirus Disease . WHO; 2021. COVID-19) Dashboard | WHO Coronavirus Disease (COVID-19) Dashboard.https://covid19.who.int/?gclid=CjwKCAjwnK36BRBVEiwAsMT8WJ3y00_BUzvrLsvbl3uthuoTH_Occ45gyEUbpYRyEqAzll3aZB6TYxoCcM0QAvD_BwE [Google Scholar]
- 2.Moderna . Inc.; Moderna: 2020. Moderna's Work on a COVID-19 Vaccine Candidate | Moderna.https://www.modernatx.com/modernas-work-potential-vaccine-against-covid-19 [Google Scholar]
- 3.Pfizer Inc. vols. 1–3. 2020. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-announce-vaccine-candidate-against (Pfizer and BioNTech Announce Vaccine Candidate against COVID-19 Achieved Success in First Interim Analysis from Phase 3 Study | Pfizer, Pfizer). [Google Scholar]
- 4.Calina D., Sarkar C., Arsene A.L., Salehi B., Docea A.O., Mondal M., Islam M.T., Zali A., Sharifi-Rad J. Recent advances, approaches and challenges in targeting pathways for potential COVID-19 vaccines development. Immunol. Res. 2020:1. doi: 10.1007/s12026-020-09154-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scudellari M. How the pandemic might play out in 2021 and beyond. Nature. 2020 doi: 10.1038/d41586-020-02278-5. [DOI] [PubMed] [Google Scholar]
- 6.WHO . World Health Organization; 2020. SARS-CoV-2 Variant – United Kingdom of Great Britain and Northern Ireland.http://www.who.int/csr/don/21-december-2020-sars-cov2-variant-united-kingdom/en/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.U.S. CDC . U.S. Centers Dis. Control Prev; 2020. Implications of the Emerging SARS-CoV-2 Variant VOC 202012/01.https://www.cdc.gov/coronavirus/2019-ncov/more/scientific-brief-emerging-variant.html [Google Scholar]
- 8.Holcombe M., Mascarenhas L. Cnn; 2020. US Coronavirus News: UK Variant of Coronavirus Found in Colorado Man, Governor Says - CNN.https://www.cnn.com/2020/12/29/health/us-coronavirus-tuesday/index.html [Google Scholar]
- 9.Guardian The. 2020. South African Covid-19 Variant May Be “More Effective at Spreading,” Guard.https://www.theguardian.com/world/2020/dec/23/south-african-covid-19-variant-may-be-more-effective-at-spreading [Google Scholar]
- 10.Fox News . Fox News; 2020. New Coronavirus Variant Appears to Emerge in Nigeria, CDC Says.https://www.foxnews.com/health/africa-cdc-new-virus-variant-appears-emerge-nigeria-africaa [Google Scholar]
- 11.Wei J., Li Y. Airborne spread of infectious agents in the indoor environment. Am. J. Infect. Contr. 2016;44:S102–S108. doi: 10.1016/j.ajic.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morawska L., Milton D.K. It is time to Address airborne transmission of coronavirus disease 2019 (COVID-19) Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson N., Corbett S., Tovey E. Airborne transmission of covid-19. BMJ. 2020:370. doi: 10.1136/bmj.m3206. [DOI] [PubMed] [Google Scholar]
- 14.Miller S.L., Nazaroff W.W., Jimenez J.L., Boerstra A., Buonanno G., Dancer S.J., Kurnitski J., Marr L.C., Morawska L., Noakes C. Transmission of SARS-CoV-2 by inhalation of respiratory aerosol in the Skagit Valley Chorale superspreading event. MedRxiv. 2020 doi: 10.1101/2020.06.15.20132027. 2020.06.15.20132027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y., Qian H., Hang J., Chen X., Hong L., Liang P., Li J., Xiao S., Wei J., Liu L., Kang M. Evidence for probable aerosol transmission of SARS-CoV-2 in a poorly ventilated restaurant. MedRxiv. 2020 doi: 10.1101/2020.04.16.20067728. 2020.04.16.20067728. [DOI] [Google Scholar]
- 16.Luo K., Lei Z., Hai Z., Xiao S., Rui J., Yang H., Jing X., Wang H., Xie Z., Luo P., Li W., Li Q., Tan H., Xu Z., Yang Y., Hu S., Chen T. Transmission of SARS-CoV-2 in public transportation vehicles: a case study in hunan province, China. Open Forum Infect. Dis. 2020;7 doi: 10.1093/ofid/ofaa430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen Y., Li C., Dong H., Wang Z., Martinez L., Sun Z., Handel A., Chen Z., Chen E., Ebell M.H., Wang F., Yi B., Wang H., Wang X., Wang A., Chen B., Qi Y., Liang L., Li Y., Ling F., Chen J., Xu G. Community outbreak investigation of SARS-CoV-2 transmission among bus Riders in eastern China. JAMA Intern. Med. 2020 doi: 10.1001/jamainternmed.2020.5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park S.Y., Kim Y.M., Yi S., Lee S., Na B.J., Kim C.B., Il Kim J., Kim H.S., Kim Y.B., Park Y., Huh I.S., Kim H.K., Yoon H.J., Jang H., Kim K., Chang Y., Kim I., Lee H., Gwack J., Kim S.S., Kim M., Kweon S., Choe Y.J., Park O., Park Y.J., Jeong E.K. Coronavirus disease outbreak in call center, South Korea. Emerg. Infect. Dis. 2020;26:1666–1670. doi: 10.3201/eid2608.201274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Setti L., Passarini F., De Gennaro G., Barbieri P., Perrone M.G., Borelli M., Palmisani J., Di Gilio A., Piscitelli P., Miani A. Airborne transmission route of COVID-19: why 2 meters/6 feet of inter-personal distance could not Be enough. Int. J. Environ. Res. Publ. Health. 2020;17:2932. doi: 10.3390/ijerph17082932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The Lancet Respiratory Medicine COVID-19 transmission—up in the air. Lancet Respir. Med. 2020;8:1159. doi: 10.1016/s2213-2600(20)30514-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis D. Mounting evidence suggests coronavirus is airborne - but health advice has not caught up. Nature. 2020 doi: 10.1038/d41586-020-02058-1. [DOI] [PubMed] [Google Scholar]
- 22.WHO . 2020. Transmission of SARS-CoV-2 : Implications for Infection Prevention Precautions.https://www.who.int/publications/i/item/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations [Google Scholar]
- 23.WHO . World Heal. Organ.; 2020. Coronavirus Disease (COVID-19): How Is it Transmitted?https://www.who.int/news-room/q-a-detail/q-a-how-is-covid-19-transmitted Coronavirus disease (COVID-19) pandemic. [Google Scholar]
- 24.U.S. CDC . U.S. Centers Dis. Control Prev.; 2020. How Coronavirus Spreads | CDC; p. 1.https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/how-covid-spreads.html%0Ahttps://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/how-covid-spreads.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fprepare%2Ftra [Google Scholar]
- 25.Fennelly K.P. Particle sizes of infectious aerosols: implications for infection control. Lancet Respir. Med. 2020;8:914–924. doi: 10.1016/S2213-2600(20)30323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prather B.K.A., Wang C.C., Schooley R.T. Reducing transmission of SARS-CoV-2. Science. 2020;368(80):1422–1424. doi: 10.1126/science.abc6197. [DOI] [PubMed] [Google Scholar]
- 27.Asadi S., Bouvier N., Wexler A.S., Ristenpart W.D. The coronavirus pandemic and aerosols: does COVID-19 transmit via expiratory particles? Aerosol Sci. Technol. 2020;54:635–638. doi: 10.1080/02786826.2020.1749229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swinkels K. Google Sheet; 2020. SARS-CoV-2 Superspreading Events Database.https://docs.google.com/spreadsheets/d/1c9jwMyT1lw2P0d6SDTno6nHLGMtpheO9xJyGHgdBoco/edit#gid=1812932356 [Google Scholar]
- 29.Byambasuren O., Cardona M., Bell Phd K., Ba J.C., Mclaws M.-L., Glasziou P. Estimating the extent of asymptomatic COVID-19 and its potential for community transmission: systematic review and meta-analysis. Off. J. Assoc. Med. Microbiol. Infect. Dis. Canada. COVID-19. 2020 doi: 10.3138/jammi-2020-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He X., Lau E.H.Y., Wu P., Deng X., Wang J., Hao X., Lau Y.C., Wong J.Y., Guan Y., Tan X., Mo X., Chen Y., Liao B., Chen W., Hu F., Zhang Q., Zhong M., Wu Y., Zhao L., Zhang F., Cowling B.J., Li F., Leung G.M. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat. Med. 2020 doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y., He Y., Tong J., Qin Y., Xie T., Li J., Li J., Xiang J., Cui Y., Higgs E.S., Xiang J. Characterization of an asymptomatic cohort of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infected individuals outside of wuhan, China. Clin. Infect. Dis. 2020;71 doi: 10.1093/cid/ciaa629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.ASHRAE . ASHRAE; 2020. ASHRAE Issues Statements on Relationship between COVID-19 and HVAC in Buildings.https://www.ashrae.org/about/news/2020/ashrae-issues-statements-on-relationship-between-covid-19-and-hvac-in-buildings [Google Scholar]
- 33.PHAC, Coronavirus disease (COVID-19 . Public Heal. Agency Canada; 2020. Prevention and Risks - Canada.Ca.https://www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection/prevention-risks.html#self [Google Scholar]
- 34.deCastro B.R., Sax S.N., Chillrud S.N., Kinney P.L., Spengler J.D. Modeling time-location patterns of inner-city high school students in New York and Los Angeles using a longitudinal approach with generalized estimating equations. J. Expo. Sci. Environ. Epidemiol. 2007;17:233–247. doi: 10.1038/sj.jes.7500504. [DOI] [PubMed] [Google Scholar]
- 35.Hussein T., Paasonen P., Kulmala M. Activity pattern of a selected group of school occupants and their family members in Helsinki — Finland. Sci. Total Environ. 2012;425:289–292. doi: 10.1016/J.SCITOTENV.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 36.Klepeis N.E., Nelson W.C., Ott W.R., Robinson J.P., Tsang A.M., Switzer P., Behar J.V., Hern S.C., Engelmann W.H. The National Human Activity Pattern Survey (NHAPS): a resource for assessing exposure to environmental pollutants. J. Expo. Sci. Environ. Epidemiol. 2001;11:231–252. doi: 10.1038/sj.jea.7500165. [DOI] [PubMed] [Google Scholar]
- 37.Schweizer C., Edwards R.D., Bayer-Oglesby L., Gauderman W.J., Ilacqua V., Juhani Jantunen M., Lai H.K., Nieuwenhuijsen M., Künzli N. Indoor time–microenvironment–activity patterns in seven regions of Europe. J. Expo. Sci. Environ. Epidemiol. 2007;17:170–181. doi: 10.1038/sj.jes.7500490. [DOI] [PubMed] [Google Scholar]
- 38.Qian H., Miao T., Liu L., Zheng X., Luo D., Li Y. Indoor transmission of SARS‐CoV‐2. Indoor Air. 2020 doi: 10.1111/ina.12766. ina.12766. [DOI] [PubMed] [Google Scholar]
- 39.Zhao L., Liu J. Operating behavior and corresponding performance of mechanical ventilation systems in Chinese residential buildings. Build. Environ. 2020;170:106600. doi: 10.1016/j.buildenv.2019.106600. [DOI] [Google Scholar]
- 40.Lu J., Gu J., Gu J., Li K., Xu C., Su W., Lai Z., Zhou D., Yu C., Xu B., Yang Z. COVID-19 outbreak associated with air conditioning in restaurant, Guangzhou, China, 2020. Emerg. Infect. Dis. 2020;26:1628–1631. doi: 10.3201/eid2607.200764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buonanno G., Morawska L., Stabile L. Quantitative assessment of the risk of airborne transmission of SARS-CoV-2 infection: prospective and retrospective applications. Environ. Int. 2020;145:106112. doi: 10.1016/j.envint.2020.106112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Balachandar S., Zaleski S., Soldati A., Ahmadi G., Bourouiba L. Host-to-host airborne transmission as a multiphase flow problem for science-based social distance guidelines. Int. J. Multiphas. Flow. 2020;132:103439. doi: 10.1016/j.ijmultiphaseflow.2020.103439. [DOI] [Google Scholar]
- 43.Zhang J. Integrating IAQ control strategies to reduce the risk of asymptomatic SARS CoV-2 infections in classrooms and open plan offices. Sci. Technol. Built Environ. 2020;26:1013–1018. doi: 10.1080/23744731.2020.1794499. [DOI] [Google Scholar]
- 44.Morawska L., Tang J.W., Bahnfleth W., Bluyssen P.M., Boerstra A., Buonanno G., Cao J., Dancer S., Floto A., Franchimon F., Haworth C., Hogeling J., Isaxon C., Jimenez J.L., Kurnitski J., Li Y., Loomans M., Marks G., Marr L.C., Mazzarella L., Melikov A.K., Miller S., Milton D.K., Nazaroff W., Nielsen P.V., Noakes C., Peccia J., Querol X., Sekhar C., Seppänen O., ichi Tanabe S., Tellier R., Tham K.W., Wargocki P., Wierzbicka A., Yao M. How can airborne transmission of COVID-19 indoors be minimised? Environ. Int. 2020;142:105832. doi: 10.1016/j.envint.2020.105832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dai H., Zhao B. Association of the infection probability of COVID-19 with ventilation rates in confined spaces. Build. Simul. 2020 doi: 10.1007/s12273-020-0703-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.WHO, Coronavirus disease (COVID-19) World Heal. Organ.; 2020. Ventilation and Air Conditioning.https://www.who.int/news-room/q-a-detail/coronavirus-disease-covid-19-ventilation-and-air-conditioning [Google Scholar]
- 47.U.S. CDC . U.S. Centers Dis. Control Prev.; 2020. Manufacturing Workers and Employers.https://search.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/en/grc-739919 [Google Scholar]
- 48.U.S. CDC . U.S. Centers Dis. Control Prev.; 2019. Long-term Care Facilities | CDC.https://www.cdc.gov/longtermcare/index.html [Google Scholar]
- 49.U.S. CDC . U.S. Centers Dis. Control Prev.; 2020. Operating Schools during COVID-19: CDC's Considerations.https://www.cdc.gov/coronavirus/2019-ncov/community/schools-childcare/schools.html [Google Scholar]
- 50.ASHRAE . 2020. Filtration/Disinfection.https://www.ashrae.org/technical-resources/filtration-disinfection [Google Scholar]
- 51.Wells W.F. Airborne contagion and air hygiene. An ecological study of droplet infections., airborne contag. Air hyg. An Ecol. Study Droplet Infect. 1955 [Google Scholar]
- 52.Riley E.C., Murphy G., Riley R.L. Airborne spread of measles in a suburban elementary school. Am. J. Epidemiol. 1978;107:421–432. doi: 10.1093/oxfordjournals.aje.a112560. [DOI] [PubMed] [Google Scholar]
- 53.Yates T.A., Khan P.Y., Knight G.M., Taylor J.G., McHugh T.D., Lipman M., White R.G., Cohen T., Cobelens F.G., Wood R., Moore D.A.J., Abubakar I. The transmission of Mycobacterium tuberculosis in high burden settings. Lancet Infect. Dis. 2016;16:227–238. doi: 10.1016/S1473-3099(15)00499-5. [DOI] [PubMed] [Google Scholar]
- 54.Qian H., Li Y., Nielsen P.V., Huang X. Spatial distribution of infection risk of SARS transmission in a hospital ward, Build. Environ. 2009;44:1651–1658. doi: 10.1016/j.buildenv.2008.11.002. [DOI] [Google Scholar]
- 55.Zemouri C., Awad S.F., Volgenant C.M.C., Crielaard W., Laheij A.M.G.A., de Soet J.J. Modeling of the transmission of coronaviruses, measles virus, influenza virus, Mycobacterium tuberculosis, and Legionella pneumophila in dental clinics. J. Dent. Res. 2020;99:1192–1198. doi: 10.1177/0022034520940288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen S.C., Liao C.M. Modelling control measures to reduce the impact of pandemic influenza among schoolchildren. Epidemiol. Infect. 2008;136:1035–1045. doi: 10.1017/S0950268807009284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stephens B. HVAC filtration and the Wells-Riley approach to assessing risks of infectious airborne diseases. NAFA Found. Rep. 2013:44. [Google Scholar]
- 58.Harrichandra A., Ierardi A.M., Pavilonis B. An estimation of airborne SARS-CoV-2 infection transmission risk in New York City nail salons. Toxicol. Ind. Health. 2020 doi: 10.1177/0748233720964650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kasibhatla P., Jimenez J., Fay J., Albright E., Pan W. 2020. COVID Exposure Modeler.http://covid-exposure-modeler-data-devils.cloud.duke.edu/ [Google Scholar]
- 60.Allen J.G., Cedeno-Laurent J., Miller S. 2020. Harvard-CU Boulder Portable Air Cleaner Calculator for Schools.v1.2.https://docs.google.com/spreadsheets/d/1NEhk1IEdbEi_b3wa6gI_zNs8uBJjlSS-86d4b7bW098/edit#gid=1882881703 [Google Scholar]
- 61.Corsi R., Van Den Wymelenberg K., Parhizkar H. 2020. Safeairspaces.https://safeairspaces.com/#bcb9fc15-e9d7-4884-876f-384f1c8418a1 [Google Scholar]
- 62.Dols William S., Polidoro B.J., Poppendieck D.G., Emmerich S.J. FaTIMA; 2020. Fate and Transport of Indoor Microbiological Aerosols.https://pages.nist.gov/CONTAM-apps/webapps/FaTIMA/ [Google Scholar]
- 63.Riediker M., Monn C. Simulation of SARS-CoV-2 aerosol emissions in the infected population and resulting airborne exposures in different indoor scenarios. Aerosol Air Qual. Res. 2020;20 doi: 10.4209/aaqr.2020.08.0531. [DOI] [Google Scholar]
- 64.REHVA . 2020. COVID-19 Ventilation Calculator.https://www.rehva.eu/covid19-ventilation-calculator [Google Scholar]
- 65.Gammaitoni L., Nucci M.C. Using a mathematical model to evaluate the efficacy of TB control measures. Emerg. Infect. Dis. 1997;3:335–342. doi: 10.3201/eid0303.970310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ko G., Thompson K.M., Nardell E.A. Estimation of tuberculosis risk on a commercial Airliner. Risk Anal. 2004;24:379–388. doi: 10.1111/j.0272-4332.2004.00439.x. [DOI] [PubMed] [Google Scholar]
- 67.Ko G., Burge H.A., Nardell E.A., Thompson K.M. Estimation of tuberculosis risk and incidence under upper room ultraviolet germicidal irradiation in a waiting room in a hypothetical scenario. Risk Anal. 2001;21:657–674. doi: 10.1111/0272-4332.214142. [DOI] [PubMed] [Google Scholar]
- 68.Carcione J.M., Santos J.E., Bagaini C., Ba J. A simulation of a COVID-19 epidemic based on a deterministic SEIR model. Front. Public Heal. 2020;8:230. doi: 10.3389/fpubh.2020.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.He S., Peng Y., Sun K. SEIR modeling of the COVID-19 and its dynamics. Nonlinear Dynam. 2020;101:1667–1680. doi: 10.1007/s11071-020-05743-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stephens B. 2012. Wells-Riley & HVAC Filtration for Infectious Airborne Aerosols NAFA Foundation Report HVAC Filtration and the Wells-Riley Approach to Assessing Risks of Infectious Airborne Diseases Final Report Prepared for: the National Air Filtration Association (NAFA)www.built-envi.com [Google Scholar]
- 71.Sze To G.N., Chao C.Y.H. Review and comparison between the Wells-Riley and dose-response approaches to risk assessment of infectious respiratory diseases. Indoor Air. 2010;20:2–16. doi: 10.1111/j.1600-0668.2009.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Riley E.C., Murphy G., Riley R.L. Airborne spread of measles in a suburban elementary school. Am. J. Epidemiol. 1978;107:421–432. doi: 10.1093/oxfordjournals.aje.a112560. [DOI] [PubMed] [Google Scholar]
- 73.Sun C., Zhai Z. The efficacy of social distance and ventilation effectiveness in preventing COVID-19 transmission. Sustain. Cities Soc. 2020;62:102390. doi: 10.1016/j.scs.2020.102390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nicas M., Nazaroff W.W., Hubbard A. Toward understanding the risk of secondary airborne infection: emission of respirable pathogens. J. Occup. Environ. Hyg. 2005;2:143–154. doi: 10.1080/15459620590918466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhao L., Qi Y., Luzzatto-Fegiz P., Cui Y., Zhu Y. COVID-19: effects of environmental conditions on the propagation of respiratory droplets. Nano Lett. 2020;20:7744–7750. doi: 10.1021/acs.nanolett.0c03331. [DOI] [PubMed] [Google Scholar]
- 76.Buonanno G., Stabile L., Morawska L. Estimation of airborne viral emission: quanta emission rate of SARS-CoV-2 for infection risk assessment. Environ. Int. 2020;141:105794. doi: 10.1016/j.envint.2020.105794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Imai N., Cori A., Dorigatti I., Baguelin M., Donnelly C.A., Riley S., Ferguson N.M. 2020. Transmissibility of 2019-nCoV. London, UK. [DOI] [Google Scholar]
- 78.Majumder M., Mandl K.D. Early transmissibility assessment of a novel coronavirus in wuhan, China. SSRN Electron. J. 2020 doi: 10.2139/ssrn.3524675. [DOI] [Google Scholar]
- 79.Read J., Bridgen J.R., Cummings D.A., Ho A., Jewell C. Novel coronavirus 2019-nCoV: early estimation of epidemiological parameters and epidemic predictions. MedRxiv. 2020 doi: 10.1101/2020.01.23.20018549. 2020.01.23.20018549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., Ren R., Leung K.S.M., Lau E.H.Y., Wong J.Y., Xing X., Xiang N., Wu Y., Li C., Chen Q., Li D., Liu T., Zhao J., Liu M., Tu W., Chen C., Jin L., Yang R., Wang Q., Zhou S., Wang R., Liu H., Luo Y., Liu Y., Shao G., Li H., Tao Z., Yang Y., Deng Z., Liu B., Ma Z., Zhang Y., Shi G., Lam T.T.Y., Wu J.T., Gao G.F., Cowling B.J., Yang B., Leung G.M., Feng Z. Early transmission dynamics in wuhan, China, of novel coronavirus–infected pneumonia. N. Engl. J. Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhao S., Lin Q., Ran J., Musa S.S., Yang G., Wang W., Lou Y., Gao D., Yang L., He D., Wang M.H. Preliminary estimation of the basic reproduction number of novel coronavirus (2019-nCoV) in China, from 2019 to 2020: a data-driven analysis in the early phase of the outbreak. Int. J. Infect. Dis. 2020;92:214–217. doi: 10.1016/j.ijid.2020.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Khalili A., Petersen E., Koopmans M., Go U., Hamer D.H., Petrosillo N., Castelli F., Storgaard M., Al Khalili S. Personal view comparing SARS-CoV-2 with SARS-CoV and influenza pandemics. Lancet Infect. Dis. 2020;20:e238–e244. doi: 10.1016/S1473-3099(20)30484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A., Niemeyer D., Jones T.C., Vollmar P., Rothe C., Hoelscher M., Bleicker T., Brünink S., Schneider J., Ehmann R., Zwirglmaier K., Drosten C., Wendtner C. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020 doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 84.Rothe C., Schunk M., Sothmann P., Bretzel G., Froeschl G., Wallrauch C., Zimmer T., Thiel V., Janke C., Guggemos W., Seilmaier M., Drosten C., Vollmar P., Zwirglmaier K., Zange S., Wölfel R., Hoelscher M. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N. Engl. J. Med. 2020;382:970–971. doi: 10.1056/nejmc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dubert M., Visseaux B., Isernia V., Bouadma L., Deconinck L., Patrier J., Wicky P.H., Le Pluart D., Kramer L., Rioux C., Le Hingrat Q., Houhou-Fidouh N., Yazdanpanah Y., Ghosn J., Lescure F.X. Case report study of the first five COVID-19 patients treated with remdesivir in France. Int. J. Infect. Dis. 2020;98:290–293. doi: 10.1016/j.ijid.2020.06.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Han M.S., Seong M.-W., Heo E.Y., Park J.H., Kim N., Shin S., Cho S.I., Park S.S., Choi E.H. Sequential analysis of viral load in a neonate and her mother infected with severe acute respiratory syndrome coronavirus 2. Clin. Infect. Dis. 2020;71:2236–2239. doi: 10.1093/cid/ciaa447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pan Y., Zhang D., Yang P., Poon L.L.M., Wang Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect. Dis. 2020;20:411–412. doi: 10.1016/S1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim J.Y., Ko J.-H., Kim Y., Kim Y.-J., Kim J.-M., Chung Y.-S., Kim H.M., Han M.-G., Kim S.Y., Chin B.S. Viral load kinetics of SARS-CoV-2 infection in first two patients in korea. J. Kor. Med. Sci. 2020;35 doi: 10.3346/jkms.2020.35.e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Morawska L., Johnson G.R., Ristovski Z.D., Hargreaves M., Mengersen K., Corbett S., Chao C.Y.H., Li Y., Katoshevski D. Size distribution and sites of origin of droplets expelled from the human respiratory tract during expiratory activities. J. Aerosol Sci. 2009;40:256–269. doi: 10.1016/j.jaerosci.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lindsley W.G., Blachere F.M., Thewlis R.E., Vishnu A., Davis K.A. Measurements of airborne influenza virus in aerosol particles from human coughs. PLoS One. 2010;5:15100. doi: 10.1371/journal.pone.0015100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.CDPH The role OF building ventilation and filtration IN reducing risk OF airborne viral transmission IN schools. ILLUSTRATED WITH SARS-COV-2. 2020 [Google Scholar]
- 92.Liu Y., Ning Z., Chen Y., Guo M., Liu Y., Gali N.K., Sun L., Duan Y., Cai J., Westerdahl D., Liu X., Xu K., fai Ho K., Kan H., Fu Q., Lan K. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020;582:557–560. doi: 10.1038/s41586-020-2271-3. [DOI] [PubMed] [Google Scholar]
- 93.Chia P.Y., Coleman K.K., Tan Y.K., Ong S.W.X., Gum M., Lau S.K., Lim X.F., Lim A.S., Sutjipto S., Lee P.H., Son T.T., Young B.E., Milton D.K., Gray G.C., Schuster S., Barkham T., De P.P., Vasoo S., Chan M., Ang B.S.P., Tan B.H., Leo Y.S., Ng O.T., Wong M.S.Y., Marimuthu K., Lye D.C., Lim P.L., Lee C.C., Ling L.M., Lee L., Lee T.H., Wong C.S., Sadarangani S., Lin R.J., Ng D.H.L., Sadasiv M., Yeo T.W., Choy C.Y., Tan G.S.E., Dimatatac F., Santos I.F., Go C.J., Chan Y.K., Tay J.Y., Tan J.Y.L., Pandit N., Ho B.C.H., Mendis S., Chen Y.Y.C., Abdad M.Y., Moses D. Detection of air and surface contamination by SARS-CoV-2 in hospital rooms of infected patients. Nat. Commun. 2020;11:1–7. doi: 10.1038/s41467-020-16670-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.ASHRAE. ASHRAE 52 . 2017. 2 Method of Testing General Ventilation Air-Cleaning Devices for Removal Efficiency by Particle Size.https://subscriptions-techstreet-com.proxy.lib.sfu.ca/products/149895 [Google Scholar]
- 95.U.S. EPA . 2011. Exposure Factors Handbook 2011 Edition (Final Report) Washington, DC. [Google Scholar]
- 96.Mueller A., Eden M., Oakes J., Bellini C., Fernandez L. Quantitative method for comparative assessment of particle removal efficiency of fabric masks as alternatives to standard surgical masks for PPE. Matter. 2020 doi: 10.1016/j.matt.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Konda A., Prakash A., Moss G.A., Schmoldt M., Grant G.D., Guha S. Aerosol filtration efficiency of common fabrics used in respiratory cloth masks. ACS Nano. 2020;14:6339–6347. doi: 10.1021/acsnano.0c03252. [DOI] [PubMed] [Google Scholar]
- 98.Chen W. 2020. The Role of Building Ventilation and Filtration in Reducing Risk of Airborne Viral Transmission in Schools , Illustrated with SARS-CoV-2. [Google Scholar]
- 99.van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I., Lloyd-Smith J.O., de Wit E., Munster V.J. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382:1564–1567. doi: 10.1056/nejmc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fears A., Klimstra W., Duprex P., Hartman A., Weaver S., Plante K., Mirchandani D., Plante J., Aguilar P., Fernandez D., Nalca A., Totura A., Dyer D., Kearney B., Lackemeyer M., Bohannon J.K., Johnson R., Garry R., Reed D., Roy C. Comparative dynamic aerosol efficiencies of three emergent coronaviruses and the unusual persistence of SARS-CoV-2 in aerosol suspensions. MedRxiv Prepr. Serv. Heal. Sci. 2020 doi: 10.1101/2020.04.13.20063784. 2020.04.13.20063784. [DOI] [Google Scholar]
- 101.Schuit M., Ratnesar-Shumate S., Yolitz J., Williams G., Weaver W., Green B., Miller D., Krause M., Beck K., Wood S., Holland B., Bohannon J., Freeburger D., Hooper I., Biryukov J., Altamura L.A., Wahl V., Hevey M., Dabisch P. Airborne SARS-CoV-2 is rapidly inactivated by simulated sunlight. J. Infect. Dis. 2020;222:564–571. doi: 10.1093/infdis/jiaa334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Smither S.J., Eastaugh L.S., Findlay J.S., Lever M.S. Experimental aerosol survival of SARS-CoV-2 in artificial saliva and tissue culture media at medium and high humidity. Emerg. Microb. Infect. 2020;9:1415–1417. doi: 10.1080/22221751.2020.1777906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dabisch P., Schuit M., Herzog A., Beck K., Wood S., Krause M., Miller D., Weaver W., Freeburger D., Hooper I., Green B., Williams G., Holland B., Bohannon J., Wahl V., Yolitz J., Hevey M., Ratnesar-Shumate S. The influence of temperature, humidity, and simulated sunlight on the infectivity of SARS-CoV-2 in aerosols. Aerosol. Sci. Technol. 2020:1–12. doi: 10.1080/02786826.2020.1829536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ilinois Department of Public Health . Public Heal; 2020. COVID-19 Outbreak Locations, Ilinois Dep.https://www.dph.illinois.gov/covid19/outbreak-locations?regionID=0&rPeriod=1 [Google Scholar]
- 105.Michigan Department of Health and Human Services . Michigan Dep. Heal. Hum. Serv; 2020. Coronavirus - OUTBREAK REPORTING.https://www.michigan.gov/coronavirus/0,9753,7-406-98163_98173_102057---,00.html [Google Scholar]
- 106.Colorado Department of Public Health&Environment, Colorado State Emergency Operations Center Outbreak data | Colorado COVID-19 updates, color. Dep. Public Heal. Color. State Emerg. Oper. Cent. 2020 https://covid19.colorado.gov/data/outbreak-data [Google Scholar]
- 107.Deru M., Field K., Studer D., Benne K., Griffith B., Torcellini P., Liu B., Halverson M., Winiarski D., Rosenberg M., Yazdanian M., Huang J., Crawley D. Publ; 2011. U.S. Department of Energy Commercial Reference Building Models of the National Building Stock; pp. 1–118. doi.org/NREL Report No. TP-5500-46861. [Google Scholar]
- 108.U.S. DOE . U.S. Dep. Energy; 2013. Commercial Prototype Building Models | Building Energy Codes Program.https://www.energycodes.gov/development/commercial/prototype_models [Google Scholar]
- 109.U.S. DOE . Department of Energy, U.S. Dep. Energy; 2020. Commercial Reference Buildings.http://energy.gov/eere/buildings/commercial-reference-buildings [Google Scholar]
- 110.Kasper M.R., Geibe J.R., Sears C.L., Riegodedios A.J., Luse T., Von Thun A.M., McGinnis M.B., Olson N., Houskamp D., Fenequito R., Burgess T.H., Armstrong A.W., DeLong G., Hawkins R.J., Gillingham B.L. An outbreak of covid-19 on an aircraft carrier. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2019375. NEJMoa2019375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xu P., Qian H., Miao T., Yen H., Tan H., Cowling B., Li Y. Transmission routes of covid-19 virus in the diamond princess cruise ship. MedRxiv. 2020 doi: 10.1101/2020.04.09.20059113. 2020.04.09.20059113. [DOI] [Google Scholar]
- 112.Zhao S., Zhuang Z., Ran J., Lin J., Yang G., Yang L., He D. The association between domestic train transportation and novel coronavirus (2019-nCoV) outbreak in China from 2019 to 2020: a data-driven correlational report. Trav. Med. Infect. Dis. 2020;33:101568. doi: 10.1016/j.tmaid.2020.101568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zheng R., Xu Y., Wang W., Ning G., Bi Y. Spatial transmission of COVID-19 via public and private transportation in China, Travel Med. Inf. Disp. 2020;34:101626. doi: 10.1016/j.tmaid.2020.101626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Moriarty L.F., Plucinski M.M., Marston B.J., Kurbatova E.V., Knust B., Murray E.L., Pesik N., Rose D., Fitter D., Kobayashi M., Toda M., Canty P.T., Scheuer T., Halsey E.S., Cohen N.J., Stockman L., Wadford D.A., Medley A.M., Green G., Regan J.J., Tardivel K., White S., Brown C., Morales C., Yen C., Wittry B., Freeland A., Naramore S., Novak R.T., Daigle D., Weinberg M., Acosta A., Herzig C., Kapella B.K., Jacobson K.R., Lamba K., Ishizumi A., Sarisky J., Svendsen E., Blocher T., Wu C., Charles J., Wagner R., Stewart A., Mead P.S., Kurylo E., Campbell S., Murray R., Weidle P., Cetron M., Friedman C.R., Behravesh C.B., Bjork A., Bower W., Bozio C., Braden Z., Bertulfo M.C., Chatham-Stephens K., Chu V., Cooper B., Dooling K., Dubray C., Curren E., Honein M.A., Ivey K., Jones J., Kadzik M., Knight N., Marlow M., McColloch A., McDonald R., Klevos A., Poser S., Rinker R.A., Ritter T., Rodriguez L., Ryan M., Schneider Z., Shockey C., Shugart J., Silver M., Smith P.W., Tobolowsky F., Treffiletti A., Wallace M., Yoder J., Barry P., Berumen R., Bregman B., Campos K., Chai S., Glenn-Finer R., Guevara H., Hacker J., Hsieh K., Morris M.K., Murphy R., Myers J.F., Padilla T., Pan C.-Y., Readhead A., Saguar E., Salas M., Snyder R.E., Vugia D., Watt J., Wong C., Acosta M., Davis S., Kapuszinsky B., Matyas B., Miller G., Ntui A., Richards J. Public health Responses to COVID-19 outbreaks on cruise ships — worldwide, february–march 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:347–352. doi: 10.15585/mmwr.mm6912e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.ASHRAE . 2020. Transportation, ASHRAE.https://www.ashrae.org/technical-resources/transportation [Google Scholar]
- 116.Khanh N.C., Thai P.Q., Quach H.L., Thi N.A.H., Dinh P.C., Duong T.N., Mai L.T.Q., Nghia N.D., Tu T.A., Quang L.N., Quang T.D., Nguyen T.T., Vogt F., Anh D.D. Transmission of SARS-CoV 2 during long-haul flight. Emerg. Infect. Dis. 2020;26:2617–2624. doi: 10.3201/eid2611.203299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Loretto . 2020. Floor Plans - Loretto.https://www.lorettocny.org/locations/cottages-garden-grove/floor-plans Loretto. [Google Scholar]
- 118.Buildings Division of the Department of Transportation and Infrastructure, DSD Design Standards for Nursing Homes. 2015. https://www2.gnb.ca/content/dam/gnb/Departments/sd-ds/pdf/NursingHomes/NursingHomeDesignStandards-e.pdf [Google Scholar]
- 119.Syracuse University Goldstein Auditorium specifications. http://scps.syr.edu/events-technical-services/goldstein-auditorium-specs.html (n.d.)
- 120.Syracuse University Floor plans - housing, meal plan, and I.D. Card services. https://housingmealplans.syr.edu/residential-facilities/floor-plans-and-room-layouts/ (n.d.)
- 121.Syracuse University Link Hall - link Hall - Answers. https://answers.syr.edu/display/itslemp/Link+Hall (n.d.)
- 122.Syracuse University Floor maps | Syracuse university libraries. https://library.syr.edu/locations/maps.php#birdmap (n.d.)
- 123.Günther T., Czech‐Sioli M., Indenbirken D., Robitaille A., Tenhaken P., Exner M., Ottinger M., Fischer N., Grundhoff A., Brinkmann M.M. SARS‐CoV‐2 outbreak investigation in a German meat processing plant. EMBO Mol. Med. 2020;12 doi: 10.15252/emmm.202013296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pang Z., Chen Y., Zhang J., O'Neill Z., Cheng H., Dong B. Nationwide HVAC energy-saving potential quantification for office buildings with occupant-centric controls in various climates. Appl. Energy. 2020;279:115727. doi: 10.1016/j.apenergy.2020.115727. [DOI] [Google Scholar]
- 125.IM P., New J.R., Bae Y. Build. Simul. 2019. 2019. Updated OpenStudio small and medium office prototype models. [Google Scholar]
- 126.Whole Building Design Guide Chapel facilities design guide. Whole Build. Des. Guid. 2020 https://www.wbdg.org/FFC/AF/AFDG/ARCHIVES/chapelfacilities.pdf [Google Scholar]
- 127.Pempus B. CardPlayer; 2020. In Las Vegas, Poker Still Beats Sports Betting in Revenue Per Square Foot.https://www.cardplayer.com/poker-news/22416-in-las-vegas-poker-still-beats-sports-betting-in-revenue-per-square-foot [Google Scholar]
- 128.Global air . 2020. Airbus A320 Specifications, Cabin Dimensions, Performance.https://www.globalair.com/aircraft-for-sale/Specifications?specid=639 [Google Scholar]
- 129.Airbus . Airbus; 2020. A320ceo - A320 Family -https://www.airbus.com/aircraft/passenger-aircraft/a320-family/a320ceo.html#details [Google Scholar]
- 130.Princess Cruises . Princess Cruises; 2020. Diamond Princess Fact Sheet,https://www.princess.com/news/backgrounders_and_fact_sheets/factsheet/Diamond-Princess-Fact-Sheet.html [Google Scholar]
- 131.Carnival Cruise Lines, Carnival Cabin Details & Selection, Carnival Cruise Lines. 2020. https://www.cruisin.me/info/carnival-cruise-lines/cabin-details-selection/ [Google Scholar]
- 132.Kawasaki Rail Car Inc. Kawasaki Rail Car Inc; 2020. New York City Transit Authority R142A.http://www.kawasakirailcar.com/R142A [Google Scholar]
- 133.Dimensions.com . Dimensions.Com; 2020. Bus Dimensions & Drawings.https://www.dimensions.com/collection/buses [Google Scholar]
- 134.Track School Bus What is the average size of a school bus? Track Sch. Bus. 2020 https://www.reference.com/science/average-size-cheek-cell-b92cec9d6cb800a5 [Google Scholar]
- 135.Melikov A.K., Ai Z.T., Markov D.G. Intermittent occupancy combined with ventilation: an efficient strategy for the reduction of airborne transmission indoors. Sci. Total Environ. 2020;744:140908. doi: 10.1016/j.scitotenv.2020.140908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Junjing Y., Sekhar C., Cheong D., Raphael B. Performance evaluation of an integrated Personalized Ventilation-Personalized Exhaust system in conjunction with two background ventilation systems. Build. Environ. 2014;78:103–110. doi: 10.1016/j.buildenv.2014.04.015. [DOI] [Google Scholar]
- 137.Dygert R.K., Dang T.Q. Mitigation of cross-contamination in an aircraft cabin via localized exhaust. Build. Environ. 2010;45:2015–2026. doi: 10.1016/j.buildenv.2010.01.014. [DOI] [Google Scholar]
- 138.Ahmed A.Q., Gao S. Numerical investigation of height impact of local exhaust combined with an office work station on energy saving and indoor environment. Build. Environ. 2017;122:194–205. doi: 10.1016/j.buildenv.2017.06.011. [DOI] [Google Scholar]
- 139.U.S. CDC Scientific brief: community use of cloth masks to control the spread of SARS-CoV-2. U.S. Centers Dis. Control Prev. 2020 https://www.cdc.gov/coronavirus/2019-ncov/more/masking-science-sars-cov2.html [PubMed] [Google Scholar]
- 140.Persily A., Gorfain J. Analysis of ventilation data from the U.S. Environmental protection Agency building assessment survey and evaluation (BASE) study. Environ. Protect. 2004 [Google Scholar]
- 141.WHO . WHO; 2020. Air Travel Advice.https://www.who.int/news-room/q-a-detail/air-travel-advice [Google Scholar]
- 142.Kong M., Zhang J., Wang J. Air and air contaminant flows in office cubicles with and without personal ventilation: a CFD modeling and simulation study. Build. Simul. 2015;8:381–392. doi: 10.1007/s12273-015-0219-6. [DOI] [Google Scholar]
- 143.Halvoňová B., Melikov A.K. Performance of “ductless” personalized ventilation in conjunction with displacement ventilation: impact of intake height. Build. Environ. 2010;45:996–1005. doi: 10.1016/j.buildenv.2009.10.007. [DOI] [Google Scholar]
- 144.Dygert R.K., Dang T.Q. Experimental validation of local exhaust strategies for improved IAQ in aircraft cabins. Build. Environ. 2012;47:76–88. doi: 10.1016/j.buildenv.2011.04.025. [DOI] [Google Scholar]
- 145.Schoen L.J. ASHRAE J; 2020. Guidance for Building Operations during the COVID-19 Pandemic.https://www.ashrae.org/file library/technical resources/ashrae journal/2020journaldocuments/72-74_ieq_schoen.pdf [Google Scholar]
- 146.Zhao B., Liu Y., Chen C. Air purifiers: a supplementary measure to remove airborne SARS-CoV-2. Build. Environ. 2020;177 doi: 10.1016/j.buildenv.2020.106918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Liu G., Xiao M., Zhang X., Gal C., Chen X., Liu L., Pan S., Wu J., Tang L., Clements-Croome D. A review of air filtration technologies for sustainable and healthy building ventilation. Sustain. Cities Soc. 2017;32:375–396. doi: 10.1016/j.scs.2017.04.011. [DOI] [Google Scholar]
- 148.AHAM Air filtration standards & sustainability, AHAM. (n.d.) https://ahamverifide.org/ahams-air-filtration-standards/
- 149.U.S. EPA . 2018. Guide to Air Cleaners in the Home. [Google Scholar]
- 150.CDC, Niosh Basic upper-room ultraviolet germicidal irradiation guidelines for healthcare settings department of health and human services centers for disease control and prevention national institute for occupational safety and health, n.d. www.cdc.gov/niosh
- 151.Xu P., Peccia J., Fabian P., Martyny J.W., Fennelly K.P., Hernandez M., Miller S.L. Efficacy of ultraviolet germicidal irradiation of upper-room air in inactivating airborne bacterial spores and mycobacteria in full-scale studies, Atmos. Environ. 2003;37:405–419. doi: 10.1016/S1352-2310(02)00825-7. [DOI] [Google Scholar]
- 152.Nicas M., Miller S.L. A multi-zone model evaluation of the efficacy of upper-room air ultraviolet germicidal irradiation. Appl. Occup. Environ. Hyg. 1999;14:317–328. doi: 10.1080/104732299302909. [DOI] [PubMed] [Google Scholar]
- 153.Beggs C.B., Noakes C.J., Sleigh P.A., Fletcher L.A., Kerr K.G. Methodology for determining the susceptibility of airborne microorganisms to irradiation by an upper-room UVGI system. J. Aerosol Sci. 2006;37:885–902. doi: 10.1016/j.jaerosci.2005.08.002. [DOI] [Google Scholar]
- 154.Kanaan M., Abou Moughlbay A. 2018. Comparative CFD Investigation of Upper Room UVGI Efficacy with Three Different Ventilation Systems.http://www.ripublication.com [Google Scholar]
- 155.Chu D.K., Akl E.A., Duda S., Solo K., Yaacoub S., Schünemann H.J., El-harakeh A., Bognanni A., Lotfi T., Loeb M., Hajizadeh A., Bak A., Izcovich A., Cuello-Garcia C.A., Chen C., Harris D.J., Borowiack E., Chamseddine F., Schünemann F., Morgano G.P., Muti Schünemann G.E.U., Chen G., Zhao H., Neumann I., Chan J., Khabsa J., Hneiny L., Harrison L., Smith M., Rizk N., Giorgi Rossi P., AbiHanna P., El-khoury R., Stalteri R., Baldeh T., Piggott T., Zhang Y., Saad Z., Khamis A., Reinap M. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395:1973–1987. doi: 10.1016/S0140-6736(20)31142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.McKinsey & Company . McKinsey Co.; 2020. Survey: in Thed US, People Say Their Use of Masks May Endure.https://www.mckinsey.com/featured-insights/americas/survey-in-the-us-people-say-their-use-of-masks-may-endure# [Google Scholar]
- 157.Forbes . 2021. Germany Mandates Medical-Grade Masks, Forbes.https://www.forbes.com/sites/tommybeer/2021/01/20/germany-mandates-medical-grade-masks/?sh=6e43a6622596 [Google Scholar]
- 158.U.S. CDC . U.S. Centers Dis. Control Prev.; 2020. COVID-19: Considerations for Wearing Masks.https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/cloth-face-cover-guidance.html [Google Scholar]
- 159.WHO . 2020. Weekly Operational Update on COVID-19.http://bit.ly/infodemiologycall [Google Scholar]
- 160.Johns Hopkins COVID-19 map - johns hopkins coronavirus resource center, johns hopkins coronavirus Resour. Cent. 2020:1. https://coronavirus.jhu.edu/data/mortality%0Ahttps://coronavirus.jhu.edu/map.html [Google Scholar]
- 161.The New York Times . The New York Times; New York Times: 2020. Coronavirus in the U.S.: Latest Map and Case Count; pp. 1–23.https://www.nytimes.com/interactive/2020/us/coronavirus-us-cases.html [Google Scholar]
- 162.U.S. CDC . Centers Dis. Control Prev.; 2020. COVID-19 Pandemic Planning Scenarios. [Google Scholar]
- 163.Rothaman K.J., Greenland S., Lash T.L. 2008. Modern Epidemiology.https://books.google.mv/books?hl=en&lr=&id=Z3vjT9ALxHUC&oi=fnd&pg=PR7&ots=aSFG9HQO20&sig=4YqQ4njkepTyhkyHJxYHLvg7YyA&redir_esc=y#v=onepage&q&f=false [Google Scholar]
- 164.ECDC . 2020. COVID-19 in Children and the Role of School Settings in COVID-19 Transmission. [Google Scholar]
- 165.WHO . 2020. What We Know about COVID-19 Transmission in Schools. [Google Scholar]
- 166.Blinder A., Higgins L., Guggenheim B. New York Times; 2020. College Sports Has Reported at Least 6,629 Virus Cases.https://www.nytimes.com/2020/12/11/sports/coronavirus-college-sports-football.html [Google Scholar]
- 167.Hubler S., Hartocollis A. New York Times; 2020. How Campuses Became the New Covid-19 Hotspots.https://www.nytimes.com/2020/09/11/us/college-campus-outbreak-covid.html [Google Scholar]
- 168.Diep F. 2020. The 5 Biggest Lessons We’ve Learned about How Coronavirus Spreads on Campus.https://www.chronicle.com/article/the-5-biggest-lessons-weve-learned-about-how-coronavirus-spreads-on-campus?bc_nonce=0psvozh8g0wdhazw9aarviv&cid=reg_wall_signup [Google Scholar]
- 169.Wilson E., Donovan C.V., Campbell M., Chai T., Pittman K., Seña A.C., Pettifor A., Weber D.J., Mallick A., Cope A., Porterfield D.S., Pettigrew E., Moore Z. Multiple COVID-19 clusters on a university campus — North Carolina, August 2020, MMWR. Morb. Mortal. Wkly. Rep. 2020;69:1416–1418. doi: 10.15585/mmwr.mm6939e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Teran R.A., Ghinai I., Gretsch S., Cable T., Black S.R., Green S.J., Perez O., Chlipala G.E., Maienschein-Cline M., Kunstman K.J., Bleasdale S.C., Fricchione M.J. COVID-19 outbreak among a university's men's and women's soccer teams — chicago, Illinois, july–August 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:1591–1594. doi: 10.15585/mmwr.mm6943e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lewis M. COVID-19 outbreak among college students after a spring break trip to Mexico — Austin, Texas, march 26–April 5, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:830–835. doi: 10.15585/mmwr.mm6926e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Platsky J., Roth A.N., Taddeo S., Robinson D. USA Today; 2020. COVID-19 Ravaged These New York Factories. This Is How it Happened.https://www.uticaod.com/story/news/2020/07/23/covid-19-ravaged-these-new-york-factories-this-is-how-it-happened/113423838/ [Google Scholar]
- 173.Waltenburg M.A., Victoroff T., Rose C.E., Butterfield M., Jervis R.H., Fedak K.M., Gabel J.A., Feldpausch A., Dunne E.M., Austin C., Ahmed F.S., Tubach S., Rhea C., Krueger A., Crum D.A., Vostok J., Moore M.J., Turabelidze G., Stover D., Donahue M., Edge K., Gutierrez B., Kline K.E., Martz N., Rajotte J.C., Julian E., Diedhiou A., Radcliffe R., Clayton J.L., Ortbahn D., Cummins J., Barbeau B., Murphy J., Darby B., Graff N.R., Dostal T.K.H., Pray I.W., Tillman C., Dittrich M.M., Burns-Grant G., Lee S., Spieckerman A., Iqbal K., Griffing S.M., Lawson A., Mainzer H.M., Bealle A.E., Edding E., Arnold K.E., Rodriguez T., Merkle S., Pettrone K., Schlanger K., LaBar K., Hendricks K., Lasry A., Krishnasamy V., Walke H.T., Rose D.A., Honein M.A., Amoroso K., Diallo Y., Fazekas K., Finley P.J., Fuld J., Guest J.L., Herstein J.J., Kennedy E.D., Lawler J.V., Lowe J.J., Neifert A., Schwedhelm M.M., Medicine N., Steinberg J.M., Trout D.B., Zarate-Bermudez M. Update: COVID-19 among workers in meat and poultry processing facilities ― United States, April–may 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:887–892. doi: 10.15585/mmwr.mm6927e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Steinberg J., Kennedy E.D., Basler C., Grant M.P., Jacobs J.R., Ortbahn D., Osburn J., Saydah S., Tomasi S., Clayton J.L. COVID-19 outbreak among employees at a meat processing facility — south Dakota, march–April 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:1015–1019. doi: 10.15585/mmwr.mm6931a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Middleton J., Reintjes R., Lopes H. Meat plants-a new front line in the covid-19 pandemic. BMJ. 2020;370 doi: 10.1136/bmj.m2716. [DOI] [PubMed] [Google Scholar]
- 176.Stewart A., Kottasova I., Khaliq A. Cnn; 2020. Why Meat Processing Plants Have Become Covid-19 Hotbeds.https://www.cnn.com/2020/06/27/health/meat-processing-plants-coronavirus-intl/index.html [Google Scholar]
- 177.Guenther T., Czech-Sioli M., Indenbirken D., Robitailles A., Tenhaken P., Exner M., Ottinger M., Fischer N., Grundhoff A., Brinkmann M. Investigation of a superspreading event preceding the largest meat processing plant-related SARS-Coronavirus 2 outbreak in Germany. SSRN Electron. J. 2020 doi: 10.2139/ssrn.3654517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Richards S., Vassalos M. COVID-19 Amplifies local meat supply chain issues in South Carolina. J. Agric. Food Syst. Community Dev. 2020;10:1–5. doi: 10.5304/jafscd.2020.101.001. [DOI] [Google Scholar]
- 179.BBC News . BBC News; 2020. 2 Sisters Anglesey: 158 Factory Staff Have Coronavirus.https://www.bbc.com/news/uk-wales-53131765 [Google Scholar]
- 180.BBC News . BBC News; 2020. Coronavirus: Hundreds of Abattoir Workers Test Positive in Germany.https://www.bbc.com/news/world-europe-53087139 [Google Scholar]
- 181.Wallace M., Hagan L., Curran K.G., Williams S.P., Handanagic S., Bjork A., Davidson S.L., Lawrence R.T., McLaughlin J., Butterfield M., James A.E., Patil N., Lucas K., Hutchinson J., Sosa L., Jara A., Griffin P., Simonson S., Brown C.M., Smoyer S., Weinberg M., Pattee B., Howell M., Donahue M., Hesham S., Shelley E., Philips G., Selvage D., Staley E.M., Lee A., Mannell M., McCotter O., Villalobos R., Bell L., Diedhiou A., Ortbahn D., Clayton J.L., Sanders K., Cranford H., Barbeau B., McCombs K.G., Holsinger C., Kwit N.A., Pringle J.C., Kariko S., Strick L., Allord M., Tillman C., Morrison A., Rowe D., Marlow M. COVID-19 in correctional and detention facilities — United States, february–April 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:587–590. doi: 10.15585/mmwr.mm6919e1. [DOI] [PubMed] [Google Scholar]
- 182.Njuguna H., Wallace M., Simonson S., Tobolowsky F.A., James A.E., Bordelon K., Fukunaga R., Gold J.A.W., Wortham J., Sokol T., Haydel D., Tran H., Kim K., Fisher K.A., Marlow M., Tate J.E., Doshi R.H., Curran K.G. Serial laboratory testing for SARS-CoV-2 infection among incarcerated and detained persons in a correctional and detention facility — Louisiana, April–may 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:836–840. doi: 10.15585/mmwr.mm6926e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wallace M., Marlow M., Simonson S., Walker M., Christophe N., Dominguez O., Kleamenakis L., Orellana A., Pagan-Pena D., Singh C., Pogue M., Saucier L., Lo T., Benson K., Sokol T. Public health response to COVID-19 cases in correctional and detention facilities — Louisiana, march–April 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:594–598. doi: 10.15585/mmwr.mm6919e3. [DOI] [PubMed] [Google Scholar]
- 184.Hagan L.M., Williams S.P., Spaulding A.C., Toblin R.L., Figlenski J., Ocampo J., Ross T., Bauer H., Hutchinson J., Lucas K.D., Zahn M., Chiang C., Collins T., Burakoff A., Bettridge J., Stringer G., Maul R., Waters K., Dewart C., Clayton J., de Fijter S., Sadacharan R., Garcia L., Lockett N., Short K., Sunder L., Handanagic S. Mass testing for SARS-CoV-2 in 16 prisons and jails — six jurisdictions, United States, April–may 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:1139–1143. doi: 10.15585/mmwr.mm6933a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Davlantes E., Toro M., Villalobos R., Sanchez-Gonzalez L. Notes from the field: COVID-19 prevention practices in state prisons — Puerto Rico. MMWR Morb. Mortal. Wkly. Rep. 2020;69(2020):1144. doi: 10.15585/mmwr.mm6933a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Council of Europe Prisons and prisoners in europe in pandemic times – council of europe Annual penal statistics. Counc. Eur. Annu. Penal Stat. 2020 https://wp.unil.ch/space/publications/2199-2/ [Google Scholar]
- 187.Fisher K.A., Tenforde M.W., Feldstein L.R., Lindsell C.J., Shapiro4 N.I., Files D.C., Gibbs K.W., Erickson H.L., Prekker M.E. Community and close contact exposures associated with COVID-19 among symptomatic adults ≥18 Years in 11 outpatient health care facilities — United States, july 2020. Cent. Dis. Control Prev. MMWR. 2020;69 doi: 10.15585/mmwr.mm6936a5. https://www.cdc.gov/phlp/publications/topic/resources/legalepimodel/index.html [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.