FIGURE 2.
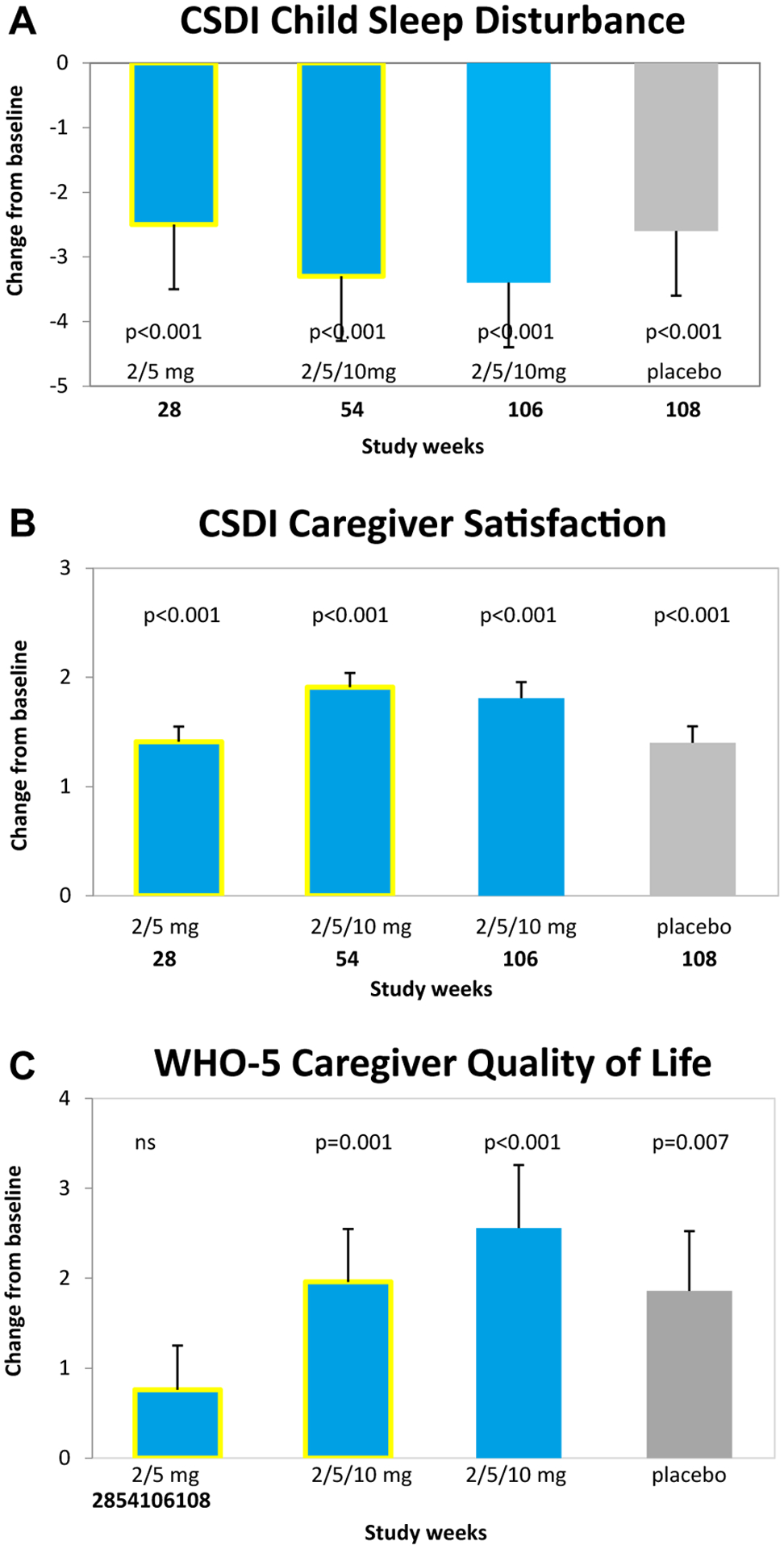
Effects of Pediatric Prolonged-Release Melatonin (PedPRM) Treatment on Child and Caregiver Parameters
Note: Effects of PedPRM (2, 5, or 10 mg/day) for 28 weeks,15 54 weeks,15 and 106 weeks and placebo withdrawal (2 weeks) on (A) CSDI child sleep disturbance, (B) CSDI caregiver satisfaction, and (C) WHO-5 caregiver quality of life. Data from 106 weeks and 2 weeks withdrawal are mean (SE) change from baseline in the combined PedPRM and placebo population. CSDI = Composite Sleep Disturbance Index; PSQI = Pittsburgh Sleep Quality Index; WHO-5 = 5-item World Health Organization Well-Being Index.
