Figure 1. Fork stalling at structure forming repeats results in repeat fragility and instability.
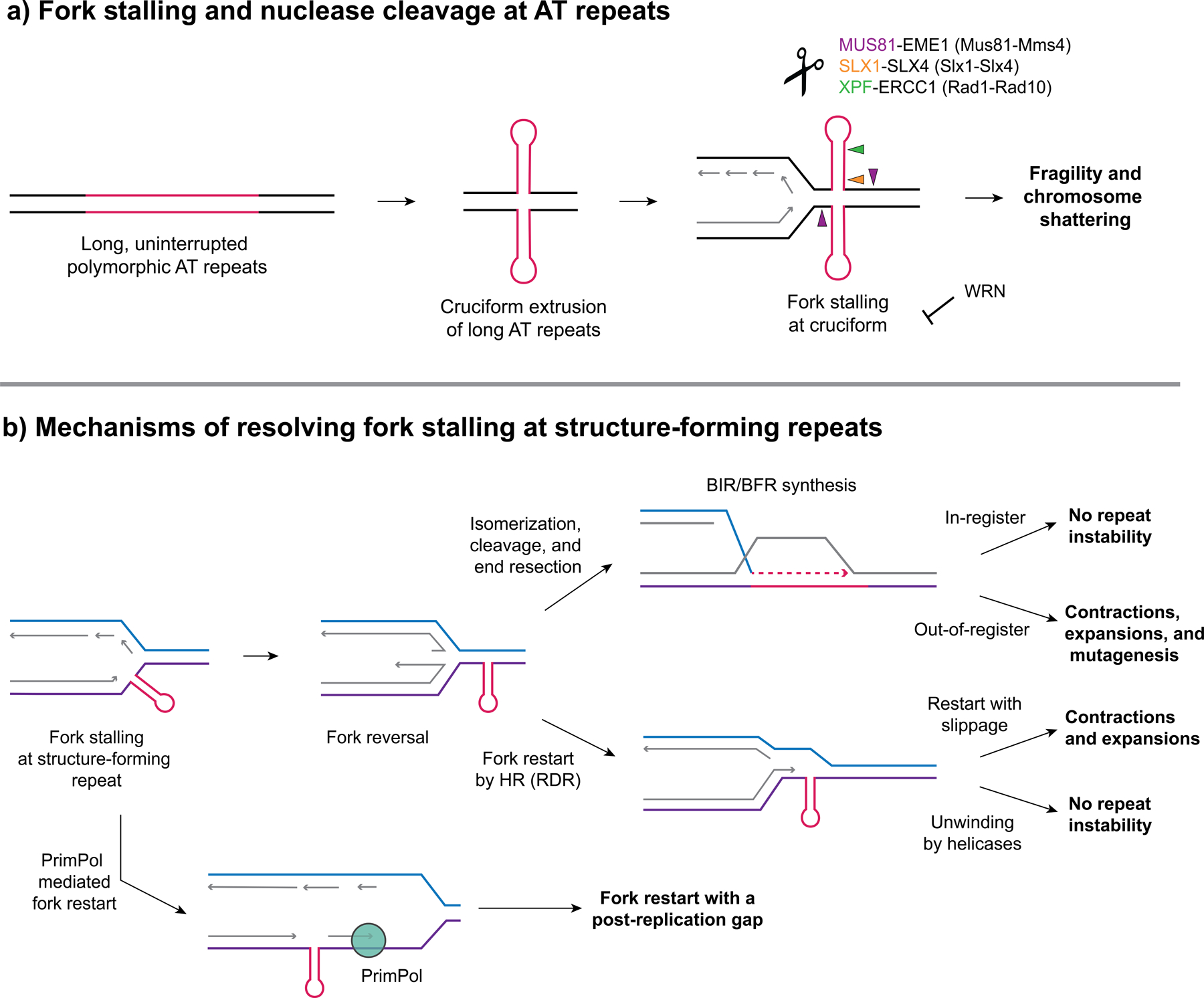
(a) Long, uninterrupted polymorphic AT repeats have the potential to form cruciform structures that serve as a barrier to replication and cause fork stalling and ATR activation. WRN (Werner Syndrome) helicase (a RecQ helicase) can be recruited to unwind the structure and prevent fork collapse. Loss of WRN results in chromosome shattering in MMR deficient MSI cancers with expanded AT repeats. (b) Pathways to resolve fork stalling at structure-forming repeats (a hairpin is shown but it could also be a G4 or triplex structure). Fork stalling can occur due to structure-forming repeats serving as a barrier to replication on either the leading or lagging strand and can result in fork reversal (a resected reversed fork is shown). Fork restart can occur through several pathways: (1) repriming past the structure, e.g. by PrimPol (2) through recombination-dependent replication (RDR), using the displaced 3’ end from a reversed fork and template strand invasion, and (3) through a BIR-like pathway after fork cleavage and end resection (referred to as broken fork repair, BFR). These pathways can result in expansions or contractions if slippage or out-of-register invasion or structure bypass occurs. Alternatively, unwinding of the structure by helicases during restart can avoid repeat instability. Exposed ssDNA accumulating during BIR can result in repeat-induced mutagenesis (RIM).
