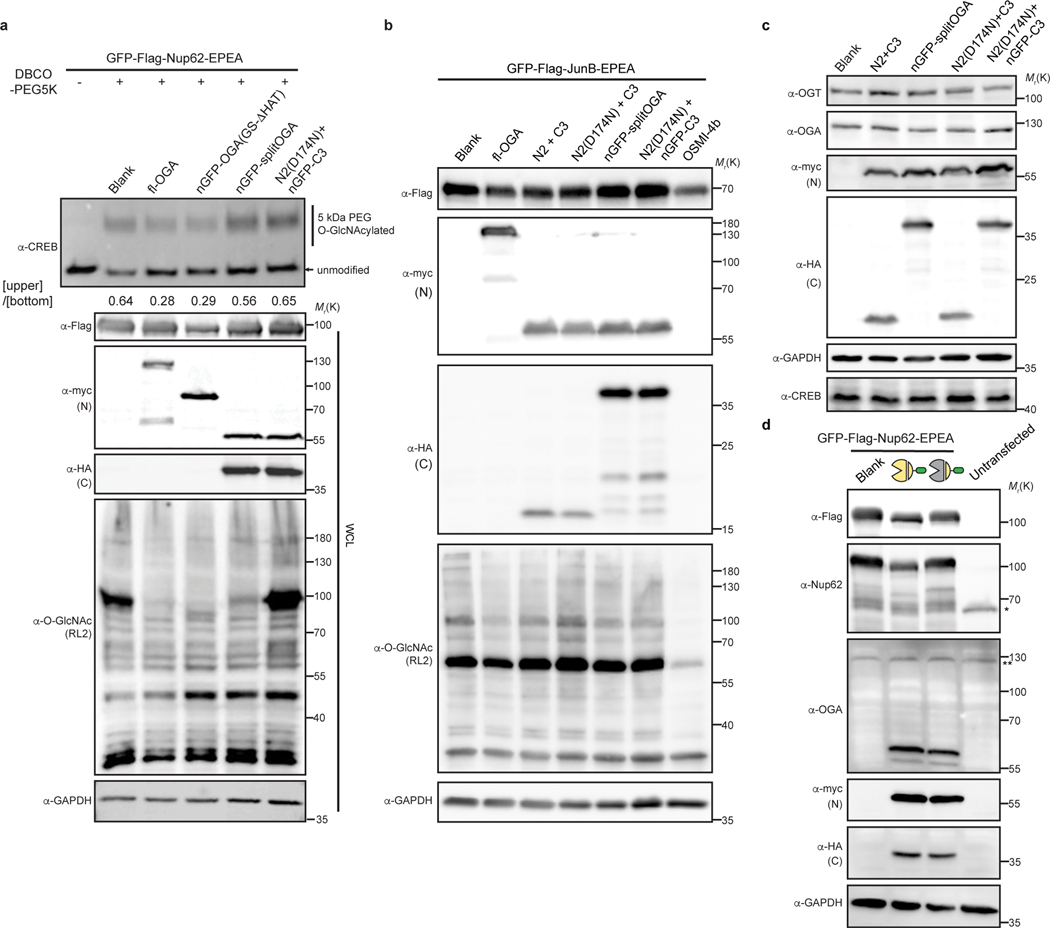
Extended Data Fig. 5. nGFP-splitOGA selectively deglycosylates the target protein without affecting the global O-GlcNAc proteome.
a, nGFP-splitOGA has little effect on endogenous glycoprotein CREB. HEK 293T cells co-expressing OGA constructs with GFP-Nup62 were subjected to mass shift assay. The intensities of O-GlcNAcylated and unmodified CREB were quantified. The ratios are shown below the anti-CREB blot. WCL, whole cell lysates. b, Overexpression of selected split OGA constructs with target protein has little effect on global O-GlcNAcylation level. For OGT inhibition, cells were treated with 25 μM OSMI-4b for 30 h. Global O-GlcNAcylation level was evaluated by anti-O-GlcNAc (RL2) antibody. c, nGFP-splitOGA has minimal effect on protein levels of endogenous OGT, OGA and glycoprotein CREB. Anti-myc and anti-HA blots detect expression of N-terminal fragment, and C-terminal fragment, respectively. d, Comparison of overexpressed proteins with the corresponding endogenous proteins. The antibody against OGA recognizes both endogenous OGA and the overexpressed N-terminal fragment of split OGA. Endogenous Nup62(*) and OGA (**) are indicated. The data in a-d are representative of at least two biological replicates.