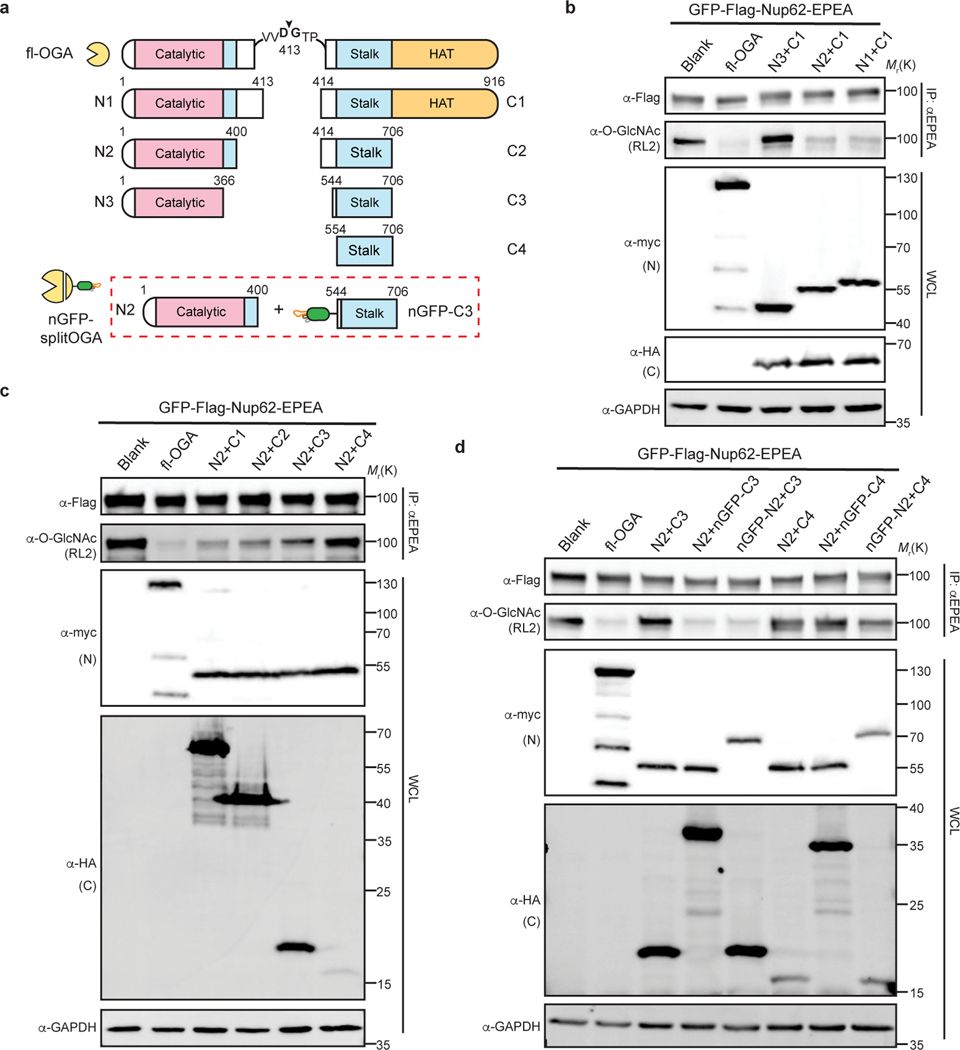
Fig. 2 |. Design and optimization of nanobody-fused split OGA for protein-selective deglycosylation on GFP-Nup62.
a, Schematic of the split site, N-fragments, and C-fragments tested in this study. Amino acid numbers appear on top. The optimal combination N2 with nGFP-fused C3 (shown in red rectangle) is termed nGFP-splitOGA. b, Optimization of the N-fragment with C1 on GFP-Nup62 to obtain a minimal N-fragment. c, Optimization of the C-fragment with N2 on GFP-Nup62 to obtain a minimal C-fragment with limited inherent substrate activity. d, nGFP fusion to split OGA regains activity on GFP-Nup62. The optimized split OGA (N2 + C3) shows limited substrate activity that is reinstated after fusion with nGFP on either fragment. GFP-Nup62 was co-expressed with the indicated constructs, enriched by anti-EPEA beads, and analyzed by immunoblotting to reveal the protein level and O-GlcNAc modification level respectively. WCL, whole cell lysate. The data in b-d are representative of two biological replicates.