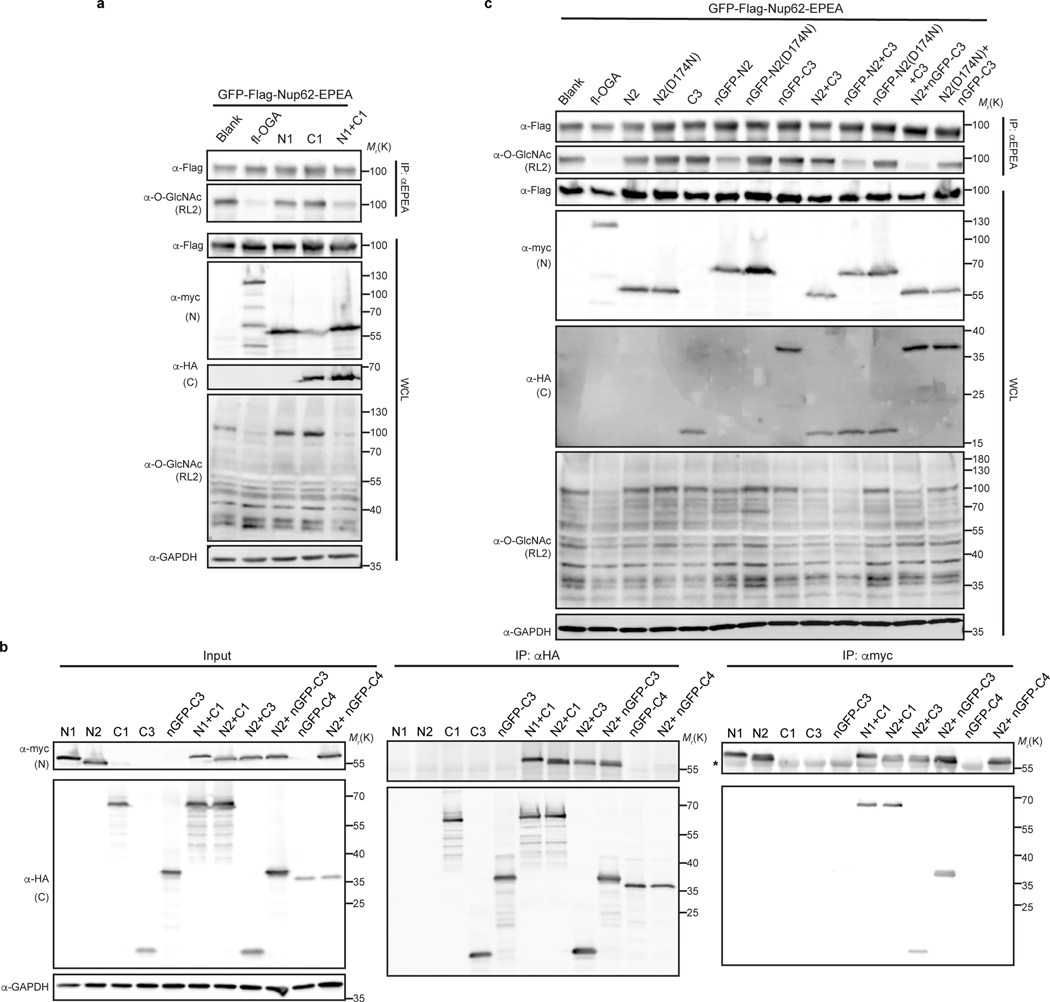
Extended Data Fig. 4. Optimization of nGFP-fused split OGA constructs in living cells.
a, Co-expressing N- and C-fragments of OGA reconstitutes deglycosidase activity in HEK 293T cells. b, Split OGA fragments, N2 and C3, instead of C4, associate with each other when co-expressed in HEK 293T cells. The asterisk indicated IgG heavy chain from anti-c-Myc magnetic beads. c, Comparison of nGFP-fused N- and C-terminal OGA fragments on GFP-Nup62 in HEK 293T cells. The pair of N2 and nGFP-fused C3 (N2 + nGFP-C3) shows the best deglycosylation performance. In a and c, activities of fragments alone or pairs with/without nGFP were evaluated on GFP-Nup62, which was enriched by beads against EPEA tag and blotted with RL2 antibody to reveal O-GlcNAc modification level. D174N, a catalytically impaired mutation on OGA. Anti-myc and anti-HA blots detect expression of full-length (fl-OGA) or N-terminal fragment, and C-terminal fragment, respectively. WCL, whole cell lysates. The data in a-c are representative of at least two biological replicates.