Abstract
Background:
Rumination and worry are repetitive negative thinking (RNT) tendencies that contribute to the development and maintenance of internalizing psychopathologies. Accruing data suggest rumination and worry represent overlapping and unique transdiagnostic cognitive processes. Yet, prior neuroimaging research has mostly focused on rumination in depression, which points to involvement of resting-state brain activity in default mode, executive, salience, and/or affective networks.
Methods:
The current study examined relations between brain activity during rest and RNT in a transdiagnostic sample. Resting-state fMRI data was analyzed in 80 un-medicated patients with internalizing conditions. Regression analysis, controlling for anxiety and depression symptoms, was performed with seed regions implicated in default mode, executive, salience, and affective networks. Rumination and worry were assessed with standard self-report measures.
Results:
Whole-brain regression results showed more rumination and worry jointly corresponded with greater positive resting-state functional connectivity (rsFC) between the amygdala and prefrontal regions (i.e., middle frontal gyrus, inferior frontal gyrus). Conversely, more worry (controlling for rumination) corresponded with greater negative rsFC between amygdala and precuneus. No significant results were observed for rumination alone (controlling for worry).
Conclusions:
Findings indicate the affective network plays a role in RNT, and distinct patterns of connectivity between amygdala and regions implicated in the executive and default mode networks were observed across patients with internalizing conditions. Results suggest different mechanisms contribute to RNT as a unitary construct and worry as a unique construct.
Keywords: fMRI, resting-state functional connectivity, rumination, worry, anxiety, depression
Introduction
It has been well documented that individuals with internalizing disorders exhibit elevated levels of rumination and worry (McEvoy et al., 2013; Newman & Llera, 2011; Nolen-Hoeksema et al., 2008). Rumination, which involves dwelling on past negative events and the possible causes and implications of negative mood (Nolen-Hoeksema, 2004), and worry, wherein thoughts are focused on uncertain and future negative events (Borkovec et al., 1983), have been traditionally examined within the context of depression (Nolen-Hoeksema et al., 2008) and anxiety (Newman & Llera, 2011), respectively. However, emerging evidence suggests that these are not fully distinct constructs (Topper et al., 2014) and may be conceptualized as transdiagnostic, dimensional, stable forms of repetitive negative thinking (RNT) (McEvoy et al., 2013; Olatunji et al., 2010; Smith & Alloy, 2009). Indeed, both shared variance between rumination and worry (i.e., RNT) and unique variance attributed to each of these constructs has been observed to correlate with symptoms of depression and anxiety (McEvoy & Brans, 2013; Spinhoven et al., 2015; Topper et al., 2014). Further, evidence that these forms of RNT are not just correlates of internalizing disorders, but also contribute to their development and maintenance (Ehring & Watkins, 2008) indicates they may serve as an important targets for intervention (Mennin & Fresco, 2013). Therefore, the delineation of mechanisms that underlie RNT may provide important insights into novel targets for intervention.
Well-established intrinsic networks support processes that are hypothesized to underlie RNT. The default mode network (DMN), which is anchored in the posterior cingulate cortex (PCC), is involved in self-referential processing (Northoff et al., 2006). The salience network, which is anchored in the dorsal anterior cingulate cortex (DACC), plays a role in the detection of external or internal salient stimuli (Seeley et al., 2007). The executive control network, which is anchored in the dorsolateral prefrontal cortex (DLPFC), supports top-down cognitive control processes (Seeley et al., 2007). Finally, the affective network, which is anchored in the amygdala, plays an important role in emotion processing (Phelps, 2006).
In light of its central role in self-referential processing (Hamilton et al., 2015; Whitfield-Gabrieli & Ford, 2012), the DMN is proposed to play a role in RNT. Other putative neural mechanisms of RNT involve interactions between bottom-up emotional reactivity and top-down inhibitory control (Disner et al., 2011; Hirsch & Mathews, 2012) that may contribute to the negative content and self-focus of RNT. Not surprisingly, seed-based resting-state studies of rumination have largely involved depressed or remitted depressed individuals with a focus on the DMN. Rumination has been shown to positively correlate with resting-state functional connectivity (rsFC) between the PCC and midline cortical regions in healthy and depressed participants (Berman et al., 2011) and negatively correlate with rsFC between these regions in patients with remitted depression (Lois & Wessa, 2016). Furthermore, Satyshur and colleagues (2018) reported that reflective rumination, thought to be a more adaptive form of rumination than the brooding component of rumination (Treynor et al., 2003), was positively associated with rsFC between the PCC and the medial prefrontal cortex (MPFC) in healthy and depressed participants. Collectively, results provide support for DMN in rumination despite inconsistencies in functional connectivity patterns.
Regarding other networks one study found that reflective rumination was negatively associated with rsFC between DACC and PCC in healthy and depressed participants (Satyshur et al., 2018); the same study also found that more brooding was associated with less rsFC between amygdala and temporal pole (Satyshur et al., 2018). Rumination as a unitary construct has been shown to positively relate to increased amygdala-PCC rsFC in healthy and remitted depressed adolescents (Peters et al., 2016). Finally, focusing on the executive network, when evaluating rumination as a unitary construct, a study that used a principal component approach demonstrated more rumination was associated with more rsFC between a cluster encompassing DLPFC and the PCC in depressed patients (Bessette et al., 2018). Another study found that in depressed adolescents, more rumination was associated with less rsFC between ACC and prefrontal regions (i.e., DLPFC, inferior frontal gyrus) (Connolly et al., 2013). Thus, findings provide accumulating evidence that rsFC involving DMN, salience, affective, and executive networks underlie rumination, though its neural signature remains unclear due to inconsistencies that may pertain to methodological differences across studies.
In contrast with rumination, less resting-state research has focused on worry. However, similar to rumination, there is evidence of DMN involvement. Studies that examined rsFC correlates of worry in DMN found more worry was associated with less rsFC between PCC and other DMN regions (i.e., MPFC, precuneus) in undergraduates (Burdwood et al., 2016) and individuals with generalized anxiety disorder (GAD) (Andreescu et al., 2014). Another study examining DMN, salience network, and affective network rsFC correlates of worry in healthy controls and patients with GAD found greater worry was associated with greater rsFC between the insula, a node of the salience network (Seeley et al., 2007), and the precuneus (controlling for rumination and anxiety symptoms) (Andreescu et al., 2015). Altogether, this limited research points to DMN and salience network involvement in worry in individuals with and without general anxiety.
Collectively, preliminary evidence based on disorder-specific studies suggests rsFC in DMN and task-positive networks contribute to rumination and worry. Yet, significant gaps remain when considering rumination and worry as transdiagnostic forms of RNT (McEvoy et al., 2013). Furthermore, given evidence of shared and unique variance between rumination and worry (McEvoy & Brans, 2013; Spinhoven et al., 2015; Topper et al., 2014), these constructs are expected to exhibit both shared RNT underpinnings and distinct rsFC patterns specific to rumination and worry. In support of shared pathways, a recent meta-analysis examining resting-state functional correlates of RNT suggests PCC engagement and frontal engagement correspond with rumination and worry (Makovac et al., 2020). This is consistent with reports that both rumination (Berman et al., 2011; Bessette et al., 2018; Lois & Wessa, 2016; Peters et al., 2016; Satyshur et al., 2018) and worry (Andreescu et al., 2014; Berman et al., 2011) are associated with PCC rsFC. Less is known about resting-state mechanisms that are specific to rumination and worry as few studies examined these forms of RNT simultaneously. However, evidence of salience network involvement in worry (controlling for rumination and anxiety) (Andreescu et al., 2015) suggests the network may be distinct to worry.
It is also important to note that although both rumination and worry are dimensional constructs, ranging from normative to pathological (Olatunji et al., 2010; Smith & Alloy, 2009), they are core processes that maintain internalizing disorders (Mansell & McEvoy, 2017) and individuals with psychopathology endorse significantly more RNT than healthy participants (Kircanski et al., 2015; Samtani et al., 2018; Wahl et al., 2019). Thus, examining individual differences in rumination and worry as they pertain to rsFC in patients has potential clinical utility. Specifically, rumination and worry are core processes that maintain psychopathology (Mansell & McEvoy, 2017) yet they are not typically assessed in clinical settings per se. Delineating intrinsic networks that underlie RNT may highlight its clinical relevance by providing insights into mechanisms of therapeutic change in the context of evidence-based treatments for internalizing psychopathologies and identify potential targets for novel interventions.
Therefore, the objective of the current study was to expand upon previous research by examining the neural correlates of rumination and worry in patients with internalizing psychopathologies. Consistent with previous research (Andreescu et al., 2014; Peters et al., 2016; Satyshur et al., 2018), we chose to focus on commonly examined nodes in DMN, salience, affective, and executive function networks (i.e., PCC, DACC, amygdala, DLPFC). We hypothesized more rumination and worry would correlate with more PCC engagement based on theory and meta-analytic findings (Makovac et al., 2020), though we did not make specific hypotheses as to rsFC connectivity patterns given inconsistent findings. We also expected worry would uniquely involve the salience network (i.e., when controlling for rumination (Andreescu et al., 2015)). Lastly, we explored possible similar and unique RNT correlates across a priori resting-state networks and hypothesized similar and distinct correlates of rsFC would be observed for rumination and worry.
Materials and Methods
Participants included 80 un-medicated treatment-seeking adults between the ages of 18 and 65 recruited as part of a study designed in accordance with the Research Domain Criteria initiative to examine mechanisms and predictors of treatment response in internalizing disorders (ClinicalTrials.gov Identifier: NCT01903447). Participants were recruited from a local mood and anxiety disorder outpatient clinic and the community. In the current study, only pre-treatment resting-state data that met quality control was used. Inclusion criteria included a total score of ≥23 on the Depression, Anxiety, and Stress Scale (DASS-21 [total possible scale range: 0 – 63]; Lovibond and Lovibond, 1995) and at least one common anxiety or depressive disorder; diagnostic comorbidity was permitted (see Table 1 for the prevalence of internalizing disorders). Of note, one patient did not meet the DASS-21 cut-point by 1 point (i.e., total score of 22). However, as the DASS-21 is a dimensional measure of psychopathology, the 1-point difference is not expected to have a substantive impact on findings. Also, the patient was diagnosed with a psychiatric disorder indicating a level of psychopathology sufficient to warrant treatment. Exclusionary criteria included treatment (psychotropic medication, psychotherapy), major medical and neurological illness, contraindications to magnetic resonance imaging (e.g., pregnancy, ferrous objects), current substance dependence (within 6 months of the study), current active suicidal ideation (within 6 months of the study), history of major psychiatric illness (e.g., bipolar disorder, schizophrenia), and cognitive dysfunction (e.g., traumatic brain injury, pervasive developmental disorder).
Table 1.
Principal and comorbid diagnoses.
| Count | Percentage | |
|---|---|---|
| PRINCIPAL DIAGNOSIS | ||
| General anxiety disorder | 33 | 41.25 |
| Social anxiety disorder | 20 | 25.00 |
| Major depressive disorder | 17 | 21.25 |
| Panic disorder | 4 | 5.00 |
| Posttraumatic stress disorder | 4 | 5.00 |
| Persistent depressive disorder | 2 | 2.50 |
| COMORBID DIAGNOSES | ||
| Social anxiety disorder | 29 | 36.30 |
| Major depressive disorder | 27 | 33.80 |
| General anxiety disorder | 25 | 31.30 |
| Panic disorder | 16 | 20.00 |
| Specific phobia | 12 | 15.00 |
| Persistent depressive disorder | 11 | 13.80 |
| Posttraumatic stress disorder | 7 | 8.80 |
| Agoraphobia | 5 | 6.30 |
| Alcohol abuse | 2 | 2.50 |
| Obsessive compulsive disorder | 2 | 2.50 |
| Two or more concurrent diagnoses | 65 | 81.25 |
The study was approved by the University of Illinois at Chicago Institutional Review Board, and informed consent was obtained from all participants. After obtaining consent, a trained master’s-level or doctorate-level clinician administered the Structured Clinical Interview (First, Williams, Karg, & Spitzer, 2015), Hamilton Depression Rating scale (HAMD; Hamilton, 1960), and Hamilton Anxiety Rating scale (HAMA; Hamilton, 1959) to assess for DSM-5 diagnoses and current symptoms. All participants were compensated for their time and all procedures complied with the Helsinki Declaration.
Repetitive Negative Thinking Measures
To measure rumination, participants were administered the Ruminative Response Scale (RRS; Nolen-Hoeksema, 1991), which is a 22-item self-report that has demonstrated good reliability and validity in previous studies (Cronbach’s αs: 0.88–0.92; Luminet, 2004). The RRS has a total possible range of 22 – 88 and higher scores denote more rumination.
Worry was assessed with the Penn State Worry Questionnaire (PSWQ; Meyer, Miller, Metzger, & Borkovec, 1990). The PSWQ is a 16-item self-report shown to have good validity and reliability (Cronbach’s αs: 0.88–0.95; Meyer et al., 1990; van Rijsoort et al., 1999). The PSWQ has a total possible range of 16 – 80 and higher scores denote more worry.
Resting-State Condition
Participants were instructed to view a crosshair centrally displayed on the blank gray screen, relax, and let their mind wander for the duration of the 8-minute scanning period. Padding with foam cushions was used to reduce head movement.
fMRI Data Acquisition and Preprocessing
Scanning during the resting-state condition was conducted on a 3 Tesla GE Discovery System (General Electric Healthcare; Waukesha, WI) with an 8-channel head coil. Functional data were acquired using gradient-echo echo planar imaging (EPI) sequence with the following parameters: TR=2 s, TE=minFull [~25 ms], flip angle=90°, FOV=22 × 22 cm2, acquisition matrix 64 × 64, 3-mm slice thickness, 44 axial slices, 180 volumes per run. For anatomical localization, a high-resolution, T1-weighted volumetric anatomical scan was acquired.
Data preprocessing and connectivity analyses were performed using the Functional Connectivity (CONN) toolbox (Whitfield-Gabrieli & Nieto-Castanon, 2012), which employs procedures from the Statistical Parametric Mapping software (SPM12; Wellcome Trust Centre for Neuroimaging, London, UK). Four initial volumes from each resting-state run were discarded to allow for T1 equilibration effects. Images were realigned to correct for motion, corrected for errors in slice timing, subjected to outlier detection, co-registered to the anatomical image, spatially transformed to standard MNI space using transformation calculated to transform the anatomical image to the MNI space, resampled to 2-mm voxels, and smoothed with an 8-mm FWHM Gaussian kernel prior to statistical analysis. For subsequent connectivity analysis, the unsmoothed images were used.
All participants tested negative on a urine toxicology screen prior to imaging and all participants were required to have no movement greater than 2-mm translation or 2 degrees rotation across the run for analysis. Effects of nuisance variables (white matter, CSF signals, and movement parameters) were reduced following the CompCor strategy (Behzadi et al., 2007) and outlier time points were regressed out; data were band-pass filtered to 0.008–0.09 Hz.
Analytic Approach
There were six a priori anatomy-based seed regions as follows: PCC, bilateral amygdala, DACC, and bilateral DLPFC to examine default mode, affective, salience, and executive networks, respectively (see Supplemental Figure 1). PCC, bilateral amygdala, and bilateral DLPFC seed regions were obtained from the FSL Harvard-Oxford Atlas, which is the default atlas integrated with the CONN toolbox. As this default atlas does not include DACC, the DACC seed region was derived from the median cingulate gyrus of the Automated Anatomical Labelling atlas. Specifically, DACC was defined as the part anterior to the y = 0 line of the median cingulate gyrus (Klumpp et al., 2017). Temporal correlations of the resting-state BOLD signal time series were examined for each seed and the rest of the brain. Since RNT is a core process that maintains internalizing disorders (Mansell & McEvoy, 2017) we wanted to identify unique variance of RNT not attributable to anxiety and depression symptoms. Accordingly, during second-level processing, a regression model comprised of rumination (RRS) and worry (PSWQ) total scores as covariates of interest and HAMA and HAMD total scores as covariates of no interest. For testing unique rsFC relationships between rumination and worry, RRS was a covariate of interest controlling for PSWQ and vice versa.
Following recent guidelines in response to concerns about false positives resulting from lenient significance thresholds (Eklund et al., 2016; Woo et al., 2014), whole-brain functional connectivity was considered significant if it exceeded adjustment for multiple comparisons across the entire brain (e.g., a whole-brain mask [volume=1,287,920 mm3]) as determined via simulation using the 3dClustSim utility (10,000 iterations; updated and ‘bug-free’ on December 2015; [https://afni.nimh.nih.gov/pub/dist/doc/program_help/3dClustSim.html]; Cox, 1996). To adjust for multiple comparisons (i.e., 6 seed regions), Bonferroni correction (0.05/6=0.008) was used to determine thresholds for significance. Therefore, significance at α<0.01 and a voxel threshold of p<0.001 was used, which yielded a minimum cluster size of 390 voxels (volume=3,120 mm3) for the regression analysis.
To illustrate the magnitude and directionality of significant effects, parameter estimates of functional connectivity (β weights, arbitrary units [a.u.]) were extracted from significant clusters and submitted to scatterplots in the Statistical Package for the Social Sciences (Chicago, IL; Version 22). Additionally, variance inflation factor (VIF) values for all covariates included in regression models were calculated to ensure that findings did not result from collinearity within the models. VIF values for covariates were as follows: HAMA=1.44, HAMD=1.44, RRS=1.14, PSWQ=1.13. Finally, sex differences have been observed for both rumination and worry (Johnson & Whisman, 2013; Robichaud et al., 2003). To evaluate if sex moderated findings, post-hoc regression analyses were conducted in SPSS. Specifically, significant rsFC extracted clusters were the dependent variable, sex (dummy coded) was entered as an independent variable in the first step of the regression, and HAMA, HAMD, RRS, PSWQ, RRS × Sex, and PSWQ × Sex were entered into the second step of the regression.
Results
Participant Characteristics
See Table 1 for patient principal and comorbid diagnoses, and Table 2 for demographic and clinical characteristics. As seen, 68.75% of patients in the current sample were female, and the average age was 26.93 (SD=7.90). Rumination (RRS) and worry (PSWQ) were positively correlated (r=.24, p=.03). Additionally, rumination was positively correlated with depression symptoms (HAMD) (r=.29, p=.01) but not anxiety symptoms (HAMA) (r=.16, p=.15), whereas worry was positive correlated with anxiety symptoms (r=.27, p=.02), but not depression symptoms (r=.13, p=.26).
Table 2.
Demographics and clinical characteristics.
| Total Participants (n=80) | |||
|---|---|---|---|
| Mean | Standard Deviation | Range | |
| DEMOGRAPHIC MEASURES | |||
| Age (years) | 26.93 | 7.90 | 18 – 50 |
| Education (years) | 16.26 | 2.94 | 12 – 26 |
| CLINICAL MEASURES | |||
| DASS-21 | 31.90 | 7.25 | 22 – 55 |
| HAMA | 17.13 | 6.64 | 6 – 41 |
| HAMD | 12.15 | 4.30 | 4 – 23 |
| PSWQ | 64.30 | 7.89 | 47 – 80 |
| RRS | 57.76 | 12.19 | 26 – 83 |
| Count | Percentage | ||
| SEX | |||
| Male | 25 | 31.25 | |
| Female | 55 | 68.75 | |
| HISPANIC ORIGIN | |||
| Hispanic or Latino | 15 | 18.75 | |
| Not Hispanic or Latino | 65 | 81.25 | |
| RACE/ETHNICITY | |||
| White | 45 | 56.25 | |
| Black | 15 | 18.75 | |
| Asian | 12 | 15.00 | |
| American Indian or Alaskan Native | 3 | 3.75 | |
| More than one race | 1 | 1.25 | |
| Other | 4 | 5.00 | |
Note: DASS-21 = Depression, Anxiety, and Stress Scale; HAMA = Hamilton Anxiety Rating Scale; HAMD = Hamilton Depression Rating Scale; PSWQ = Penn State Worry Questionnaire; RRS = Ruminative Response Scale.
Whole-Brain Regression
In the regression model where both rumination (RRS) and worry (PSWQ) were covariates of interest (i.e., shared neural processes model), whole-brain results controlling for symptom severity (HAMA, HAMD) indicated that more rumination and worry were associated with greater positive functional connectivity between left amygdala and a large region (peak [−44, 16, 14], k=572 voxels, z=4.25, volume=4,576 mm3, p< 0.001) primarily composed of left middle frontal gyrus (k=354 voxels) (i.e., DLPFC) extending to the triangular portion of the left inferior frontal gyrus (IGF; k=130 voxels). To illustrate the relationship for worry (r=.46) and rumination (r=.27) as one construct, see scatterplot (Figure 1). Additionally, post-hoc analyses testing for potential sex differences showed no main effect of sex or interactions between sex and PSWQ or RRS (all ps ≥.07).
Figure 1.
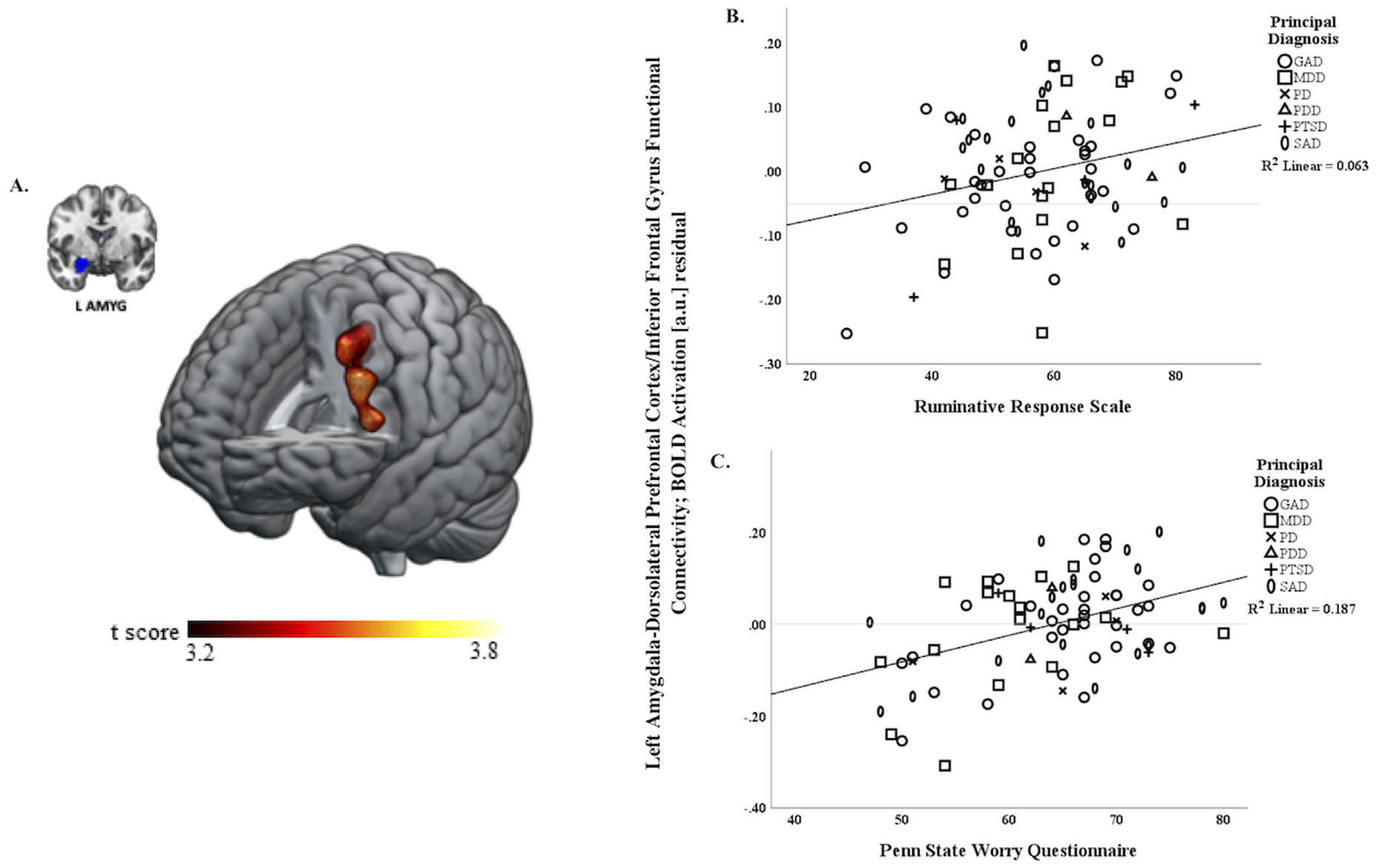
A) Whole-brain analysis of covariance with rumination assessed with the Ruminative Response Scale (RRS) and worry assessed with the Penn State Worry Questionnaire (PSWQ) as covariates of interest showing left amygdala-left dorsolateral prefrontal cortex extending to left inferior frontal gyrus (DLPFC/IFG) parameter estimates of functional connectivity, controlling for symptom severity with the Hamilton Depression Rating scale (HAMD) and Hamilton Anxiety Rating scale (HAMA) on a statistical t-map at p<0.001. B) Scatterplot of regression analyses depicting extracted parameter estimates of left amygdala-DLPFC/IFG and relationship to RRS, controlling for HAMA and HAMD, illustrating greater connectivity is associated with more rumination (higher RRS total scores). C) Scatterplot of regression analyses depicting extracted parameter estimates of left amygdala-DLPFC/IFG and relationship to PSWQ, controlling for HAMA and HAMD, showing greater connectivity is associated with more worry (higher PSWQ total scores).
In the regression model examining unique rsFC correlates of worry (PSWQ), whole brain regression analyses controlling for rumination (RRS) and symptom severity (HAMA, HAMD) revealed more worry (PSWQ) was associated with more negative functional connectivity between right amygdala and a cluster (peak [−12, −62, 26], k=828 voxels, z=4.27, volume=6,624 mm3, p<0.001) primarily composed of left precuneus (k=249 voxels) extending to right precuneus (k=219 voxels) and left median cingulate and paracingulate gyri (k=111 voxels). For depiction of the association with worry (r=−.52), see scatterplot (Figure 2). A similar finding was identified with left amygdala as the seed region, wherein greater PSWQ was associated with greater negative functional connectivity between left amygdala and a cluster (peak [2, −42, 48], k=311 voxels, z=3.92, volume=2,488 mm3, p<0.001) primarily composed of bilateral precuneus and left median cingulate and paracingulate gyri; however, the association was at a non-significant trend level (i.e., α<0.03 and voxel level p<0.001) and the number of contiguous voxels was less than the 390 threshold for significance. Post-hoc analyses indicated that patient sex, either by itself or interaction with PSWQ, was not significantly associated right amygdala-precuneus rsFC or left amygdala-precuneus rsFC (all ps ≥ .15).
Figure 2.
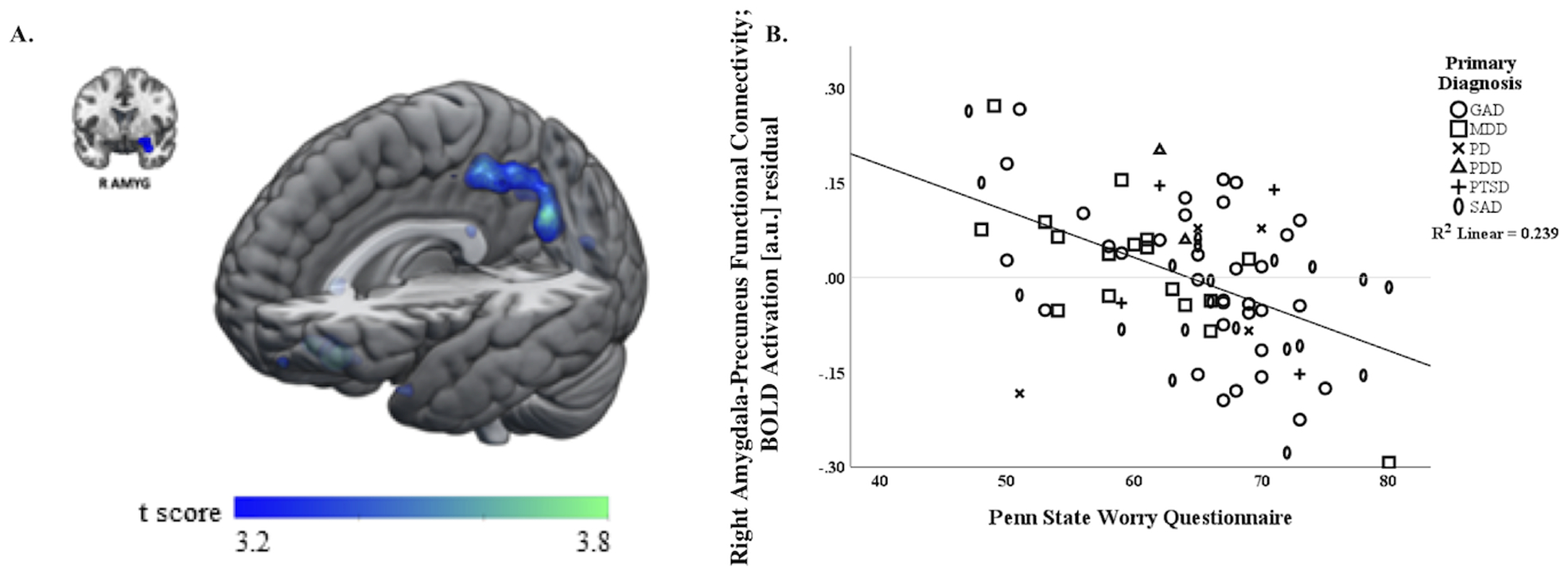
A) Whole-brain analysis of covariance with worry assessed with the Penn State Worry Questionnaire (PSWQ) as the covariate of interest controlling for rumination indexed with the Ruminative Response scale (RRS) and symptom severity assessed with the Hamilton Anxiety Rating Scale (HAMA) and Hamilton Depression Rating scale (HAMD), showing right amygdala-precuneus parameter estimates of functional connectivity on a statistical t-map at p<0.001. B) Scatter plot of the regression analyses depicting extracted parameter estimates of right amygdala-precuneus functional connectivity, controlling for rumination (RRS) and symptom severity (HAMA, HAMD), illustrating more negative connectivity is associated with more worry (higher PSWQ total scores).
Note: circles = principal generalized anxiety disorder, squares = principal major depressive disorder, x’s = principal panic disorder, triangles = principal persistent depressive disorder, crosses = posttraumatic stress disorder, ellipses = principal social anxiety disorder.
Finally, regression models examining unique rsFC correlates of rumination, controlling for PSWQ and symptoms, did not yield any significant patterns of rsFC for any a priori seed region.
Discussion
The current study is the first to our knowledge to examine rsFC correlates of rumination and worry in a transdiagnostic sample of patients with internalizing psychopathologies. Whole brain regression results, controlling for symptom severity, revealed more rumination and worry were associated with more positive rsFC between the amygdala and a cluster comprised of prefrontal regions implicated in the executive control network. Additionally, greater worry, but not rumination, was uniquely associated with more negative rsFC between the amygdala and a DMN cluster encompassing precuneus. Post-hoc analysis indicated sex did not interact with findings. Results provide preliminary evidence of shared and unique patterns of rsFC that may support repetitive negative thinking (RNT) in internalizing psychopathologies.
Evidence of both shared and unique correlates of RNT are consistent with hypotheses though significant findings were limited to when the amygdala was used as a seed region, a structure central to emotion processing, the mediation of fear responses (LeDoux, 2000), and emotional arousal (Phelps & LeDoux, 2005). Specifically, more rumination and worry were associated with greater positive rsFC between the left amygdala a cluster of prefrontal regions implicated in the executive control network, including the DLPFC and IFG. The DLPFC and IFG play important roles in top-down functions such as emotion regulation and executive processes that support regulation and other goal-oriented behavior (Banich, 2009; Disner et al., 2011; Goghari & MacDonald, 2009; Miyake & Friedman, 2012; Swick et al., 2008).
The finding that greater rumination and worry corresponded with increased amygdala-DLPFC/IFG connectivity suggests over-engagement of the executive control network may underlie RNT, regardless of content. Findings are in keeping with cognitive models of rumination and worry that posit impairment in executive and cognitive processes underlie RNT (Beckwé et al., 2014; Disner et al., 2011; Yang et al., 2017). Furthermore, findings are consistent with contemporary models that suggest RNT is an emotion regulation strategy deployed in response to emotional distress to avoid or suppress emotional experiences (Borkovec et al., 2004; Mennin et al., 2002). Thus, increased affective-executive control network functional connectivity may be indicative of increased deployment of cognitive control to engage in RNT and attempts to suppress emotional response. However, it is also possible that the relationship between RNT and affective-executive rsFC is bidirectional such that increased RNT may also lead to increased regulatory cross-talk between affective and executive networks in the attempt to modulate RNT. While further study is necessary to clarify the mechanism of this relationship, results suggest interaction between affective and executive control networks underlies rumination and worry as a unitary construct.
When examining rumination and worry separately, significant findings were detected for worry such that more worry corresponded to greater negative rsFC between the right amygdala and a cluster of regions implicated in the DMN, including bilateral precuneus. The precuneus plays an important role in integrating internal, self-generated information and externally driven information (for review see Cavanna & Trimble, 2006), and, indeed, is proposed to play a central role in mediating self-referential thought as part of the DMN (Fransson & Marrelec, 2008). In healthy participants the amygdala has been shown to be negatively coupled with precuneus during rest (Roy et al., 2009) indicating down-regulation of internal negative state. Accordingly, evidence that more worry in patients corresponded with greater negative amygdala-precuneus functional connectivity suggests a pattern that represents enhanced modulation of internal state or increased self-related processing.
In contrast to our hypothesis, there was no evidence of rsFC unique to rumination. Although the lack of an affective network finding is consistent with previous rsFC studies of rumination that utilized amygdala as a seed region and reported null effects (Peters et al., 2016; Satyshur et al., 2018), we expected rumination to involve the DMN. Also, it is surprising that significant rsFC findings were limited to amygdala as the seed region, rather than the PCC, whose role in RNT has been implicated in a recent meta-analysis (Makovac et al., 2020). It is possible that our patient sample contributed to limited or null results. Although the study was in accordance with a transdiagnostic design, the majority of patients had an anxiety disorder and comorbid anxiety was prevalent in patients with principal depression, which may relate to the challenge of recruiting un-medicated individuals with depression. Thus, the preponderance of anxiety may have resulted in higher levels of worry than rumination in the current sample, as seen in Table 2, which may have obscured distinct rsFC correlates of rumination. Interestingly, the only other study to-date to examine unique rsFC correlates of RNT in a sample which excluded patients with current depression also failed to detect unique rsFC correlates of rumination (Andreescu et al., 2015). Consequently, findings may not generalize to a depression-only patient sample or samples with higher levels of rumination.
Other limitations include the modest sample size that mostly comprised females, which may have precluded our ability to detect sex effects. Therefore, it will be important to replicate results in a larger sample balanced on sex. Also, we did not obtain any information regarding what participants were thinking about during the resting period. It is possible that individuals who habitually engage in greater rumination and worry automatically engage in other forms of RNT (e.g., idiosyncratic concerns) during rest. A better understanding of what participants think about during an unstructured rest period would aid in the interpretation of current findings. We focused on certain networks, therefore, we cannot rule out the possibility that significant findings may be observed in other networks. Finally, as is conventional, the amygdala was treated as a unitary construct, which may have reduced our ability to detect other rsFC correlates of RNT that are specific to amygdala subdivisions (Roy et al., 2009). For example, emerging research highlights the role of the bed nucleus of the stria terminalis (BNST), a subdivision of the extended amygdala (Alheid & Heimer, 1988), in anticipating unpredictable threat (Avery et al., 2016), suggesting that this specific subdivision may play a role in RNT. Indeed, as task-based functional connectivity of the BNST has been observed to correlate with both rumination and worry (Naaz et al., 2018), future studies examining rsFC correlates of RNT may benefit from examining the role of this specific structure.
Conclusion
Despite limitations, preliminary results suggest there are both shared and unique patterns of rsFC that correlate with rumination and worry in internalizing disorders. Specifically, increased positive amygdala-executive rsFC may support RNT more broadly, whereas greater negative amygdala-DMN rsFC may be specific to worry. Evidence of shared and distinct neural correlates of rumination and worry dovetails nicely with previous studies that have shown that both the shared variance of RNT as well as the unique variance of rumination and worry relate to depression and anxiety (McEvoy & Brans, 2013; Spinhoven et al., 2015; Topper et al., 2014). Findings build upon the existing body of literature by highlighting rsFC correlates of rumination and worry, which have implications for neuromodulation techniques, particularly those that target prefrontal areas to reduce symptom severity (Salomons et al., 2014). Given the role of rumination and worry in the maintenance of internalizing disorders (Ehring & Watkins, 2008), results suggest successful neuromodulation or rumination-focused interventions (e.g., Watkins et al., 2007) may reduce RNT by modulating rsFC of the affective network, thereby highlighting this intrinsic network as a potential novel target for clinical intervention.
Supplementary Material
Acknowledgements.
This work was supported by NIH/NIMH R01MH101497 (KLP), in part by NIH/NIMH R01MH112705 (HK), NIH/NIMH T32MH067631 (CF), and the Center for Clinical and Translational Research (CCTS) UL1RR029879.
Footnotes
Conflicts of Interest. The authors declare no conflicts of interest.
Data Availability Statement. The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Alheid GF, & Heimer L (1988). New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: The striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience, 27(1), 1–39. 10.1016/0306-4522(88)90217-5 [DOI] [PubMed] [Google Scholar]
- Andreescu C, Mennin D, Tudorascu D, Sheu LK, Walker S, Banihashemi L, & Aizenstein H (2015). The many faces of anxiety-neurobiological correlates of anxiety phenotypes. Psychiatry Research - Neuroimaging, 234(1), 96–105. 10.1016/j.pscychresns.2015.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreescu C, Sheu LK, Tudorascu D, Walker S, & Aizenstein H (2014). The ages of anxiety - Differences across the lifespan in the default mode network functional connectivity in generalized anxiety disorder. International Journal of Geriatric Psychiatry, 29(7), 704–712. 10.1002/gps.4051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery SN, Clauss JA, & Blackford JU (2016). The human BNST: Functional role in anxiety and addiction. Neuropsychopharmacology, 41, 126–141. 10.1038/npp.2015.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banich MT (2009). Executive function: The search for an integrated account. Current Directions in Psychological Science, 18(2), 89–94. 10.1111/j.1467-8721.2009.01615.x [DOI] [Google Scholar]
- Beckwé M, Deroost N, Koster EHW, De Lissnyder E, & De Raedt R (2014). Worrying and rumination are both associated with reduced cognitive control. Psychological Research, 78(5), 651–660. 10.1007/s00426-013-0517-5 [DOI] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, & Liu TT (2007). A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage, 37(1), 90–101. 10.1016/j.neuroimage.2007.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman MG, Peltier S, Nee DE, Kross E, Deldin PJ, & Jonides J (2011). Depression, rumination and the default network. Social Cognitive and Affective Neuroscience, 6(5), 548–555. 10.1093/scan/nsq080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessette KL, Jenkins LM, Skerrett KA, Gowins JR, DelDonno SR, Zubieta JK, McInnis MG, Jacobs RH, Ajilore O, & Langenecker SA (2018). Reliability convergent validity and time invariance of default mode network deviations in early adult major depressive disorder. Frontiers in Psychiatry, 9, 224. 10.3389/fpsyt.2018.00244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkovec TD, Alcaine OM, & Behar E (2004). Avoidance theory of worry and generalized anxiety disorder. In Heimberg RG, Turk CL, & Mennin DS (Eds.), Generalized anxiety disorder: Advances in research and practice (pp. 77–108). Guilford Press. [Google Scholar]
- Borkovec TD, Robinson E, Pruzinsky T, & DePree JA (1983). Preliminary exploration of worry: Some characteristics and processes. Behaviour Research and Therapy, 21(1), 9–16. 10.1016/0005-7967(83)90121-3 [DOI] [PubMed] [Google Scholar]
- Burdwood EN, Infantolino ZP, Crocker LD, Spielberg JM, Banich MT, Miller GA, & Heller W (2016). Resting-state functional connectivity differentiates anxious apprehension and anxious arousal. Psychophysiology, 53(10), 1451–1459. 10.1111/psyp.12696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna AE, & Trimble MR (2006). The precuneus: A review of its functional anatomy and behavioural correlates. Brain, 129(3), 564–583. 10.1093/brain/awl004 [DOI] [PubMed] [Google Scholar]
- Connolly CG, Wu J, Ho TC, Hoeft F, Wolkowitz O, Eisendrath S, Frank G, Hendren R, Max JE, Paulus MP, Tapert SF, Banerjee D, Simmons AN, & Yang TT (2013). Resting-state functional connectivity of subgenual anterior cingulate cortex in depressed adolescents. Biological Psychiatry, 74(12), 898–907. 10.1016/j.biopsych.2013.05.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW (1996). AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29(3), 162–173. 10.1006/cbmr.1996.0014 [DOI] [PubMed] [Google Scholar]
- Disner SG, Beevers CG, Haigh EAP, & Beck AT (2011). Neural mechanisms of the cognitive model of depression. Nature Reviews Neuroscience, 12(8), 467–477. 10.1038/nrn3027 [DOI] [PubMed] [Google Scholar]
- Ehring T, & Watkins ER (2008). Repetitive negative thinking as a transdiagnostic process. International Journal of Cognitive Therapy, 1(3), 192–205. 10.1521/ijct.2008.1.3.192 [DOI] [Google Scholar]
- Eklund A, Nichols TE, & Knutsson H (2016). Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences of the United States of America, 113(28), 7900–7905. 10.1073/pnas.1602413113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Williams J, Karg R, & Spitzer R (2015). Structured Clinical Interview for DSM-5-Research Version (SCID-5 for DSM-5, Research Version; SCID-5-RV). American Psychiatric Association. [Google Scholar]
- Fransson P, & Marrelec G (2008). The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: Evidence from a partial correlation network analysis. NeuroImage, 42(3), 1178–1184. 10.1016/j.neuroimage.2008.05.059 [DOI] [PubMed] [Google Scholar]
- Goghari VM, & MacDonald AW (2009). The neural basis of cognitive control: Response selection and inhibition. Brain and Cognition, 71(2), 72–83. 10.1016/j.bandc.2009.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka SM, Young CB, Klumpp H, Kennedy AE, Francis J, Ajilore O, Langenecker SA, Shankman SA, Craske MG, Stein MB, & Phan KL (2019). Emotion-based brain mechanisms and predictors for SSRI and CBT treatment of anxiety and depression: A randomized trial. Neuropsychopharmacology, 44, 1639–1648. 10.1038/s41386-019-0407-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Farmer M, Fogelman P, & Gotlib IH (2015). Depressive rumination, the default-mode network, and the dark matter of clinical neuroscience. Biological Psychiatry, 78(4), 224–230. 10.1016/j.biopsych.2015.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M (1959). The assessment of anxiety states by rating. British Journal of Medical Psychology, 32, 50–55. 10.1111/j.2044-8341.1959.tb00467.x [DOI] [PubMed] [Google Scholar]
- Hamilton M (1960). A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry, 23, 56–62. 10.1136/jnnp.23.1.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch CR, & Mathews A (2012). A cognitive model of pathological worry. Behaviour Research and Therapy, 50(10), 636–646. 10.1016/j.brat.2012.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DP, & Whisman MA (2013). Gender differences in rumination: A meta-analysis. Personality and Individual Differences, 55(4), 367–374. 10.1016/j.paid.2013.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircanski K, Thompson RJ, Sorenson JE, Sherdell L, & Gotlib IH (2015). Rumination and worry in daily life: Examining the naturalistic validity of theoretical constructs. Clinical Psychological Science, 3(6), 926–939. 10.1177/2167702614566603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpp H, Fitzgerald JM, Kinney KL, Kennedy AE, Shankman SA, Langenecker SA, & Phan KL (2017). Predicting cognitive behavioral therapy response in social anxiety disorder with anterior cingulate cortex and amygdala during emotion regulation. NeuroImage: Clinical, 15, 25–34. 10.1016/j.nicl.2017.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE (2000). Emotion circuits in the brain. Annual Review of Neuroscience, 23(1), 155–184. 10.1146/annurev.neuro.23.1.155 [DOI] [PubMed] [Google Scholar]
- Lois G, & Wessa M (2016). Differential association of default mode network connectivity and rumination in healthy individuals and remitted MDD patients. Social Cognitive and Affective Neuroscience, 11(11), 1792–1801. 10.1093/scan/nsw085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovibond PF, & Lovibond SH (1995). The structure of negative emotional states: Comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behaviour Research and Therapy, 33(3), 335–343. 10.1016/0005-7967(94)00075-U [DOI] [PubMed] [Google Scholar]
- Luminet O (2004). Measurement of depressive rumination and associated constructs. In Papageorgiou C & Wells A (Eds.), Depressive Rumination: Nature, Theory and Treatment (pp. 187–215). Wiley. 10.1002/9780470713853.ch10 [DOI] [Google Scholar]
- Makovac E, Fagioli S, Rae CL, Critchley HD, & Ottaviani C (2020). Can’t get it off my brain: Meta-analysis of neuroimaging studies on perseverative cognition. Psychiatry Research - Neuroimaging, 295, 111020. 10.1016/j.pscychresns.2019.111020 [DOI] [PubMed] [Google Scholar]
- Mansell W, & McEvoy PM (2017). A test of the core process account of psychopathology in a heterogenous clinical sample of anxiety and depression: A case of the blind men and the elephant? Journal of Anxiety Disorders, 46, 4–10. 10.1016/j.janxdis.2016.06.008 [DOI] [PubMed] [Google Scholar]
- McEvoy PM, & Brans S (2013). Common versus unique variance across measures of worry and rumination: Predictive utility and mediational models for anxiety and depression. Cognitive Therapy and Research, 37(1), 183–196. 10.1007/s10608-012-9448-5 [DOI] [Google Scholar]
- McEvoy PM, Watson H, Watkins ER, & Nathan P (2013). The relationship between worry, rumination, and comorbidity: Evidence for repetitive negative thinking as a transdiagnostic construct. Journal of Affective Disorders, 151(1), 313–320. 10.1016/j.jad.2013.06.014 [DOI] [PubMed] [Google Scholar]
- Mennin DS, & Fresco DM (2013). What, me worry and ruminate about DSM-5 and RDoC? The importance of targeting negative self-referential processing. Clinical Psychology: Science and Practice, 20(3), 258–267. 10.1111/cpsp.12038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennin DS, Heimberg RG, Turk CL, & Fresco DM (2002). Applying an emotion regulation framework to integrative approaches to generalized anxiety disorder. Clinical Psychology: Science and Practice, 9(1), 85–90. 10.1093/clipsy/9.1.85 [DOI] [Google Scholar]
- Meyer TJ, Miller ML, Metzger RL, & Borkovec TD (1990). Development and validation of the penn state worry questionnaire. Behaviour Research and Therapy, 28(6), 487–495. 10.1016/0005-7967(90)90135-6 [DOI] [PubMed] [Google Scholar]
- Miyake A, & Friedman NP (2012). The nature and organization of individual differences in executive functions: Four general conclusions. Current Directions in Psychological Science, 21(1), 8–14. 10.1177/0963721411429458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naaz F, Knight LK, & Depue BE (2018). Explicit and ambiguous threat processing: Functionally dissociable roles of the amygdala and bed nucleus of the stria terminalis. Journal of Cognitive Neuroscience, 31(4), 543–559. 10.1162/jocn_a_01369 [DOI] [PubMed] [Google Scholar]
- Newman MG, & Llera SJ (2011). A novel theory of experiential avoidance in generalized anxiety disorder: A review and synthesis of research supporting a contrast avoidance model of worry. Clinical Psychology Review, 31(3), 371–382. 10.1016/j.cpr.2011.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S (1991). Responses to depression and their effects on the duration of depressive episodes. Journal of Abnormal Psychology, 100(4), 569–582. 10.1037/0021-843X.100.4.569 [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S (2004). The response styles theory. In Papageorgiou C & Wells A (Eds.), Depressive rumination: Nature, theory and treatment. Wiley. 10.1002/9780470713853.ch6 [DOI] [Google Scholar]
- Nolen-Hoeksema S, Wisco BE, & Lyubomirsky S (2008). Rethinking rumination. Perspectives on Psychological Science, 3(5), 400–424. 10.1111/j.1745-6924.2008.00088.x [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, & Panksepp J (2006). Self-referential processing in our brain—A meta-analysis of imaging studies on the self. NeuroImage, 31(1), 440–457. 10.1016/j.neuroimage.2005.12.002 [DOI] [PubMed] [Google Scholar]
- Olatunji BO, Broman-Fulks JJ, Bergman SM, Green BA, & Zlomke KR (2010). A taxometric investigation of the latent structure of worry: Dimensionality and associations with depression, anxiety, and stress. Behavior Therapy, 41(2), 212–228. 10.1016/j.beth.2009.03.001 [DOI] [PubMed] [Google Scholar]
- Peters AT, Burkhouse K, Feldhaus CC, Langenecker SA, & Jacobs RH (2016). Aberrant resting-state functional connectivity in limbic and cognitive control networks relates to depressive rumination and mindfulness: A pilot study among adolescents with a history of depression. Journal of Affective Disorders, 200, 178–181. 10.1016/j.jad.2016.03.059 [DOI] [PubMed] [Google Scholar]
- Phelps EA (2006). Emotion and cognition: Insights from studies of the human amygdala. Annual Review of Psychology, 57, 27–53. 10.1146/annurev.psych.56.091103.070234 [DOI] [PubMed] [Google Scholar]
- Phelps EA, & LeDoux JE (2005). Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron, 48(2), 175–187. 10.1016/j.neuron.2005.09.025 [DOI] [PubMed] [Google Scholar]
- Robichaud M, Dugas MJ, & Conway M (2003). Gender differences in worry and associated cognitive-behavioral variables. Journal of Anxiety Disorders, 17(5), 501–516. 10.1016/S0887-6185(02)00237-2 [DOI] [PubMed] [Google Scholar]
- Roy AK, Shehzad Z, Margulies DS, Kelly AMC, Uddin LQ, Gotimer K, Biswal BB, Castellanos FX, & Milham MP (2009). Functional connectivity of the human amygdala using resting state fMRI. NeuroImage, 45(2), 614–626. 10.1016/j.neuroimage.2008.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomons TV, Dunlop K, Kennedy SH, Flint A, Geraci J, Giacobbe P, & Downar J (2014). Resting-state cortico-thalamic-striatal connectivity predicts response to dorsomedial prefrontal rTMS in major depressive disorder. Neuropsychopharmacology, 39, 488–498. 10.1038/npp.2013.222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samtani S, McEvoy PM, Mahoney AEJ, Werner-Seidler A, Li SSY, McGill BC, Tockar J, & Moulds ML (2018). Examining a transdiagnostic measure of repetitive thinking in depressed, formerly depressed and never-depressed individuals. Journal of Affective Disorders, 229, 515–522. 10.1016/j.jad.2017.12.081 [DOI] [PubMed] [Google Scholar]
- Satyshur MD, Layden EA, Gowins JR, Buchanan A, & Gollan JK (2018). Functional connectivity of reflective and brooding rumination in depressed and healthy women. Cognitive, Affective and Behavioral Neuroscience, 18(5), 884–901. 10.3758/s13415-018-0611-7 [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, & Greicius MD (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience, 36(2), 141–152. 10.1523/JNEUROSCI.5587-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JM, & Alloy LB (2009). A roadmap to rumination: A review of the definition, assessment, and conceptualization of this multifaceted construct. Clinical Psychology Review, 29(2), 116–128. 10.1016/j.cpr.2008.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinhoven P, Drost J, van Hemert B, & Penninx BW (2015). Common rather than unique aspects of repetitive negative thinking are related to depressive and anxiety disorders and symptoms. Journal of Anxiety Disorders, 33, 45–52. 10.1016/j.janxdis.2015.05.001 [DOI] [PubMed] [Google Scholar]
- Swick D, Ashley V, & Turken AU (2008). Left inferior frontal gyrus is critical for response inhibition. BMC Neuroscience, 9(1), 102. 10.1186/1471-2202-9-102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topper M, Molenaar D, Emmelkamp PMG, & Ehring T (2014). Are rumination and worry two sides of the same coin? A structural equation modelling approach. Journal of Experimental Psychopathology, 5(3), 363–381. 10.5127/jep.038813 [DOI] [Google Scholar]
- Treynor W, Gonzalez R, & Nolen-Hoeksema S (2003). Rumination reconsidered: A psychometric analysis. Cognitive Therapy and Research, 27(3), 247–259. 10.1023/A:1023910315561 [DOI] [Google Scholar]
- van Rijsoort S, Emmelkamp P, & Vervaeke G (1999). The Penn State Worry Questionnaire and the Worry Domains Questionnaire: Structure, reliability and validity. Clinical Psychology & Psychotherapy: An International Journal of Theory & Practice, 6(4), 297–307. [DOI] [Google Scholar]
- Wahl K, Ehring T, Kley H, Lieb R, Meyer A, Kordon A, Heinzel CV, Mazanec M, & Schönfeld S (2019). Is repetitive negative thinking a transdiagnostic process? A comparison of key processes of RNT in depression, generalized anxiety disorder, obsessive-compulsive disorder, and community controls. Journal of Behavior Therapy and Experimental Psychiatry, 64, 45–53. 10.1016/j.jbtep.2019.02.006 [DOI] [PubMed] [Google Scholar]
- Watkins E, Scott J, Wingrove J, Rimes K, Bathurst N, Steiner H, Kennell-Webb S, Moulds M, & Malliaris Y (2007). Rumination-focused cognitive behaviour therapy for residual depression: A case series. Behaviour Research and Therapy, 45(9), 2144–2154. 10.1016/j.brat.2006.09.018 [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, & Ford JM (2012). Default mode network activity and connectivity in psychopathology. Annual Review of Clinical Psychology, 8, 49–76. 10.1146/annurev-clinpsy-032511-143049 [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, & Nieto-Castanon A (2012). Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity, 2(3), 125–141. 10.1089/brain.2012.0073 [DOI] [PubMed] [Google Scholar]
- Woo CW, Krishnan A, & Wager TD (2014). Cluster-extent based thresholding in fMRI analyses: Pitfalls and recommendations. NeuroImage, 91, 412–419. 10.1016/j.neuroimage.2013.12.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Cao S, Shields GS, Teng Z, & Liu Y (2017). The relationships between rumination and core executive functions: A meta-analysis. Depression and Anxiety, 34(1), 37–50. 10.1002/da.22539 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


