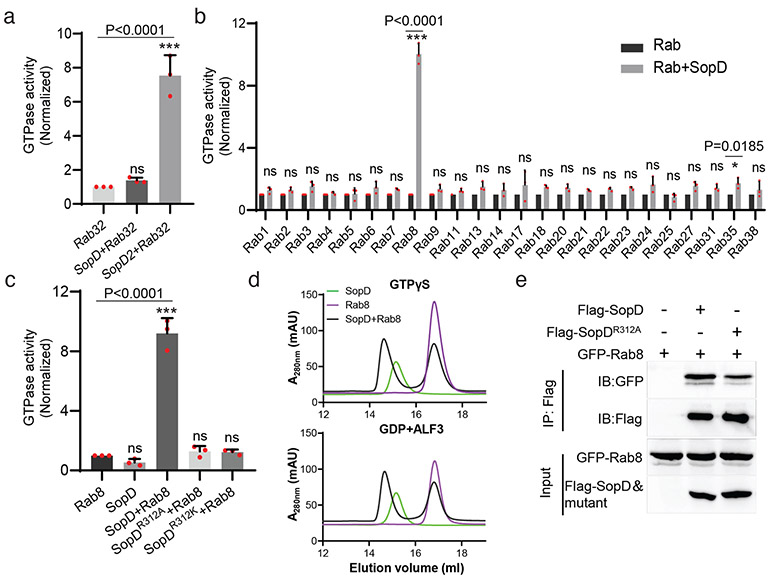
Figure 1. SopD is a GAP for Rab8.
(a) SopD lacks GAP activity toward Rab32. Purified Rab32 was incubated alone or in the presence of purified SopD or SopD2, as indicated, and the intrinsic GTPase activity was measured as indicated in Methods. (b) GAP activity of SopD toward Rab-family GTPases. Purified Rab GTPases were incubated alone or in the presence of purified SopD, and the intrinsic GTPase activity was measured as indicated in Methods. (c) SopD GAP activity toward Rab8 requires a critical arginine residue. Purified Rab81-183 was incubated alone or in the presence of purified SopD or the indicated mutants and the intrinsic GTPase activity was measured as indicated in Methods. Values in (a) (b) and (c) represent the ratio of GTPase activity observed in the presence or absence of SopD and are the mean ± SD from three independent experiments; ns, not significant; * P < 0.05, *** P < 0.001; n. s. P >0.05 (a and c) one-way ANOVA with Dunnett’s method, (b) Two-way ANOVA using Sidak's multiple comparisons test. (d) Size-exclusion chromatography profiles of SopD/Rab8 complex. Rab81-183 preloaded with GTPγS, or GDP+AlF3 were incubated with SopD, and subsequently subjected to size-exclusion chromatography in a Superdex 200 increase column. (e) Co-immunoprecipitation to detect the interaction of SopD and SopDR312A with Rab8. HEK-293T cells were transiently co-transfected with plasmids expressing GFP-Rab8 along with plasmids expressing Flag-SopD, Flag-SopDR312A or the empty vector. Cell lysates were analyzed by co-immunoprecipitation with anti-Flag and western immunoblotting with anti-GFP and anti-Flag antibodies. Experiments in (d) and (e) were conducted at least three times with equivalent results.