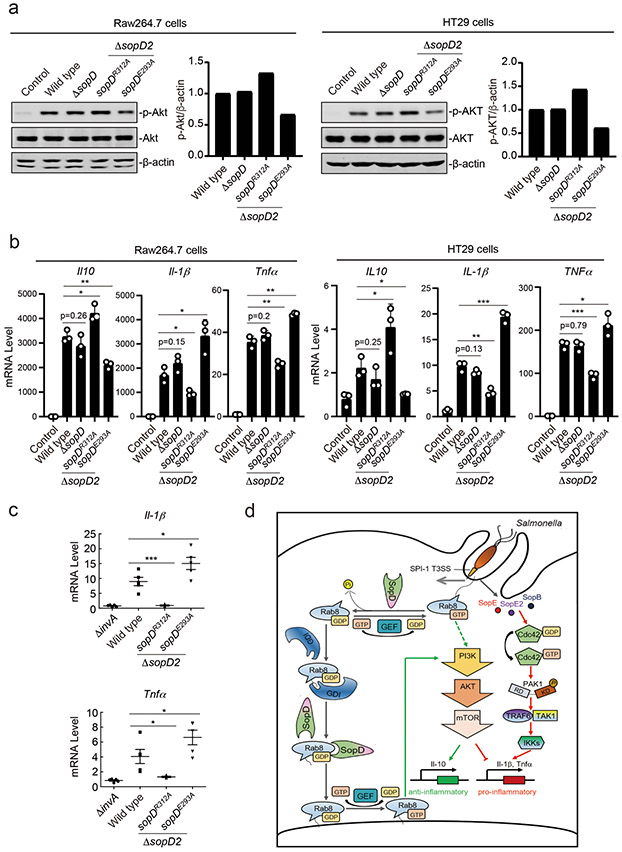
Figure 6. SopD positively and negatively modulates S. Typhimurium-induced inflammatory signaling through its independent Rab8-modulating activities.
(a and b) Akt activation and cytokine-gene expression in cells infected with S. Typhimurium strains expressing different sopD mutants. Raw264.7 (MOI=2) or HT29 (MOI=10) cells were infected with wild-type S. Typhimurium, the ΔsopD isogenic mutant, or the ΔsopD2 mutant expressing the indicated mutant alleles of sopD for 60 minutes and Akt activation was analyzed by immunoblotting with antibodies specific for the phosphorylated state of Akt and β-actin (as a loading control). The quantification of the western blots is shown in the adjacent graphs. Values are represented relative to those obtained with wild type, which have been given the arbitrary value of 1. A repetition of this experiment is shown in Supplementary Figure 10. (b) Alternatively, Raw264.7 (MOI=5) or HT29 (MOI=20) cells were infected with the same S. Typhimurium strains (as indicated) and 4 hs after infection, the mRNA levels of the indicated cytokines were quantified by qPCR. Values represent fold induction relative to uninfected controls and are the mean ± SD of three independent determinations. * P < 0.05, ** P < 0.01, *** P < 0.001, ns: not significant P > 0.05 (unpaired two-sided t test). (c) C57/BL6 nramp+/+ mice (5 animals per strain) were orally infected (~1×108 cfu) with wild-type S. Typhimurium, its isogenic ΔinvA (type III secretion defective) mutant (as a negative control), or the S. Typhimurium ΔsopD2 strains expressing the sopDR312A or sopDE293A alleles. Four days after infection the transcription levels of the indicated cytokine genes in the cells of the intestinal ceca were analyzed by qPCR assay. Values represent fold induction relative to animals infected with the S. Typhimurium ΔinvA mutant strain and are the mean ± SD. * P < 0.05, *** P < 0.001 (unpaired two-sided t test). (d) Model for SopD action during Salmonella infection. Salmonella delivers T3SS effector proteins that activate membrane ruffling, inflammatory signaling, and the activation and recruitment Rab8 to the membrane ruffles. The GAP activity of SopD reverses the activation of Rab8 thus delaying or preventing Akt-dependent anti-inflammatory signaling. Later in infection, SopD stimulates Rab8 through its GDI-displacement activity leading to the stimulation of the anti-inflammatory program.