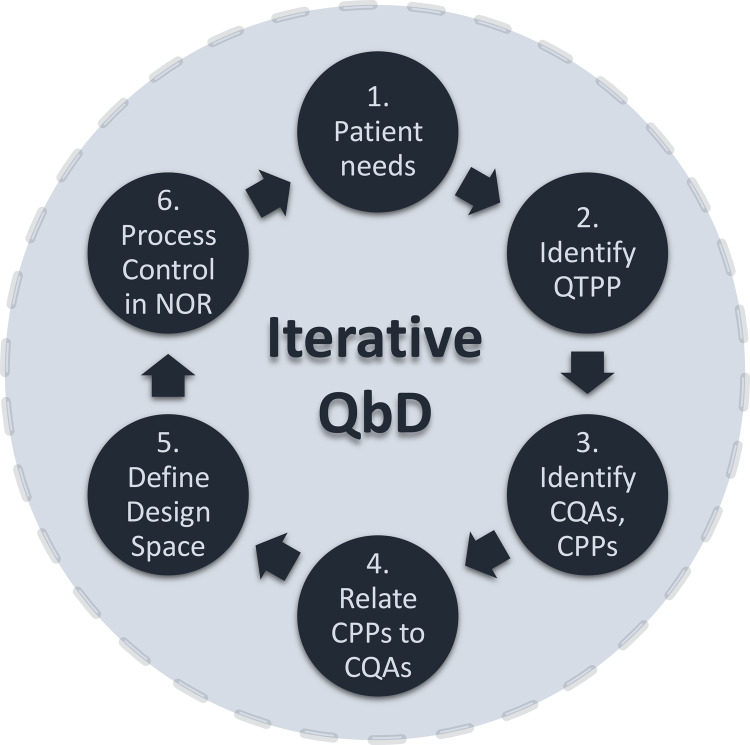
Fig. 1. Quality‐by‐design (QbD) framework development cycle.
The QbD development cycle starts with identifying the needs of the patients and from there the Quality Target Product Profile (QTPP) is determined. Based on the QTPP, the critical quality attributes (CQAs) of the product and their ranges are determined using a risk assessment scoring15, taking into account clinical and non‐clinical data, for both product safety and efficacy. Next, based on understanding how the production process unit operations impact the product CQAs, the critical process parameter (CPP) ranges are defined. From this product-process understanding, mathematical relations between CPPs and CQAs are established, thus obtaining a mathematical model of the vaccine production process at the unit operation level. This model is then used to identify the ranges of CPPs which yield the desired CQAs. From these CPP ranges, the design space can be created and therein a sub-space termed the normal operating range (NOR) is defined. The production process can be operated in NOR also by adapting the QbD bioprocess model for advanced process control, using model predictive control which takes in real‐time measurement data from the production process. The “digital twin” based automation in the NOR allows for real-time optimisation of the production process which follows current good manufacturing practices (cGMP)49,50; manufacturing products for the patients’ needs at consistently high quality. Thus, the QbD framework supports both the development and operation of production processes and it follows an iterative development cycle to ensure continuous improvement through the product‐process life cycle4,15.