Figure 2.
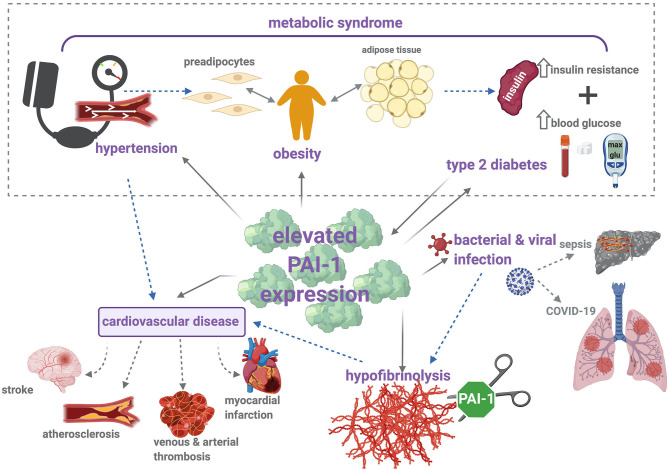
PAI-1 modulates thrombosis and inflammation via multiple pathophysiological mechanisms. Metabolic syndrome is characterized by increased insulin resistance, obesity, and hypertension, which contribute to elevated risk of cardiovascular disease (CVD). Increased levels of PAI-1 antigen and activity are positively associated with hypertension, obesity, type 2 diabetes (T2DM), and CVD. Briefly, elevated levels of PAI-1 antigen and activity occur in obesity, which is a known risk factor for CVD and T2DM. Strong correlations between increased PAI-1 and development of T2DM have been identified, including increased insulin resistance and impaired glucose tolerance. Conversely, insulin and glucose can stimulate PAI-1 secretion from adipose tissue. Elevated levels of PAI-1 attenuate plasmin formation and downregulate fibrin degradation. This hypofibrinolytic state provokes thromboembolic complications, including stroke, atherosclerosis, myocardial infarction, and venous and arterial thrombosis. Hypofibrinolysis and elevated PAI-1 levels have been associated with bacterial and viral infections, including sepsis and COVID-19. Sepsis is characterized by the development of disseminated intravascular coagulation (DIC), a major contributor to resulting organ failure. In COVID-19 patients, disruption between coagulation and fibrinolysis leads to fibrin deposits in the lung parenchyma and thrombosis. Solid arrows represent (patho)physiological processes that arise as a result of increased PAI-1 levels, and dotted arrows illustrate the links between each of these individual pathologies.