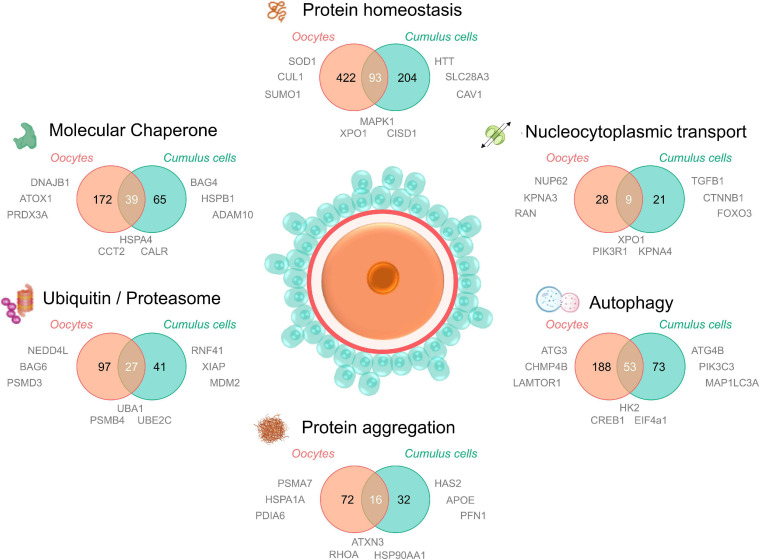
FIGURE 3.
Proteomic/transcriptomic evidence for the dysregulation of the protein homeostasis network in aged oocytes and cumulus cells. To understand the contribution of proteostasis to aging in oocytes and cumulus cells, four recent proteomic and/or transcriptomic datasets of maternally aged oocytes and cumulus cells (Oocytes; Schwarzer et al., 2014; Duncan et al., 2017; Cumulus cells; McReynolds et al., 2012; Al-Edani et al., 2014) were analyzed for dysregulated elements involved in proteostasis. Proteins and transcripts were defined as “age-dysregulated” by individual cut offs (fold change and significance) specified by the authors of each primary research paper. A database of all proteostasis network related proteins was compiled from neXtProt (Zahn-Zabal et al., 2020; v2.27.0; https://www.nextprot.org; neXtProt Data release 17/01/2020) through six individual searches titled “Protein homeostasis, Protein aggregation, Autophagy, Nucleocytoplasmic transport, Ubiquitin/proteasome, and Molecular chaperone.” Human neXtProt protein accessions were then mapped to their UniProt mouse homologs to generate both mouse and human proteostasis databases (found in Supplementary File 1). Age-dysregulated proteins and transcripts listed in the Supplementary Material of the four primary manuscripts were uniformly converted to their corresponding mouse homologs and UniProt protein accessions and used to search for corresponding proteostasis related ID’s in the 6 NeXtProt database categories. These data were used to generate Venn diagrams comparing the putative age-dysregulated proteins in oocytes vs. cumulus cells and to supply relevant examples of dysregulated proteins adjacent to the component of interest. Aged oocyte IDs were from CBCF1 mice aged between 14 and 17 months (climacteric) and aged cumulus cell IDs were from cumulus-oocyte complexes from women of equivalent advanced maternal age (37–45 years). Examples included in Figure 3 were selected from age-dysregulated protein IDs. Full protein names, UniProt accessions, the proteostasis network database used to generate this figure, and lists of oocyte and cumulus cell dysregulated protein accessions can be found in Supplementary File 1. The sharing of IDs between aged oocytes and cumulus cells is putative but provides novel candidates for research into the contribution of declining proteostasis to reproductive aging. Elements of this figure were made in BioRender.